
p53-regulated microRNA determines the fate of colorectal cancer cells under hypoxia
Colorectal cancer (CRC) patients with distant metastasis suffer from poorer prognosis as compared with those without, necessitating identification of novel preventive or therapeutic targets for metastasis. Epithelial-mesenchymal transition (EMT) is considered to play a major role in invasion and metastasis of solid cancers including CRC, while mesenchymal-epithelial transition (MET), the reverse of EMT, is associated with their reduced invasion ability (1,2). EMT is regulated primarily by so-called EMT transcription factors (EMT-TFs) including SNAI1/2, ZEB1, and TWIST1, but its complex regulatory networks include microRNAs and splicing factors that affect EMT-TFs and/or their targets (1,2). For example, miR-34a and miR-200c target SNAI1and ZEB1, respectively, and thereby regulate EMT of CRC cells (3-5). Most, if not all, of solid cancers including CRCs contain hypoxic regions, and hypoxic responses mediated chiefly by hypoxia-inducible factors (HIFs) play important roles in supporting tumor growth by turning on an angiogenic switch through induction of VEGFs and other angiogenic factors (6), as well as by inducing a metabolic reprogramming known as Warburg’s effect (7). HIFs are also involved in invasive growth and metastasis of cancers of epithelial origin by promoting EMT (8). However, hypoxia can also induce expression of the tumor suppressor p53 (9). The loss of p53, frequently found in CRCs, can thus confer a selective advantage under hypoxia, yet how the p53 status affects hypoxia-induced EMT of CRCs has been largely unknown.
The recent report by Li et al. beautifully demonstrated that hypoxia selectively stimulates EMT and thereby promotes invasion and metastasis of p53-deficient CRC cells, but not that of p53-proficient CRC cells, through HIF1A-mediated repression of miR-34a (Figure 1) (10). The authors fist showed that culturing p53-deficent CRC cells under hypoxic condition (0.5% O2) for 30 hours repressed mature miR-34a expression in a HIF1A-dependent manner, while the same treatment on p53-proficent CRC cells induced the expression of mature miR-34a. miR-34a encodes a p53-inducible microRNA (11) and intriguingly, p53-mediated induction of miR-34a appeared to be dominant over HIF1A-mediated repression of miR-34a. Ectopic expression of miR-34a in p53-deficient DLD-1 cells suppressed the levels of EMT markers and the invasion activity induced by hypoxia, indicating that HIF1A-mediated downregulation of miR-34a is required for hypoxia-induced EMT in p53-deficent CRC cells.
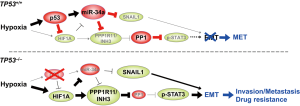
The authors next identified the mRNA encoding protein phosphatase 1 regulatory inhibitor subunit 11 (PPP1R11), also called INH3 (12), as a direct and conserved target of miR-34a. As its name indicates, PPP1R11 can inhibit PP1, the phosphatase that can dephosphorylate STAT3 at serine 727 (13,14), a phosphorylation site known to enhance homodimerization and transcriptional activity of STAT3 (15). They then showed that the PPP1R11 gene promoter contains 5 HIF1A binding sites and its expression can be induced directly by HIF1A, suggesting a coherent feed-forward regulation of PPP1R11 by HIF1A and miR-34a under hypoxia. The authors subsequently showed that PPP1R11 is necessary for EMT, migration, and invasion of DLD-1 and HT-29 cells, and that overexpression of PPP1R11 is sufficient for inducing EMT, migration, and invasion in DLD-1 cells. In addition to these in vitro results, they showed that knocking down PPP1R11 in DLD-1 cells pretreated with a hypoxic culture condition inhibits their lung metastasis upon tail-vein injection, demonstrating that PPP1R11 mediates hypoxia-induced metastasis of p53-deficient CRC cells. Negative regulation of PPP1R11 and the EMT phenotypes by miR-34a in vivo was further demonstrated using genetically-engineered mouse models of early-stage colorectal carcinogenesis. Namely, intestinal tumors from miR-34a−/−;miR-34bc−/−;ApcMin/+ compound mutant mice showed elevated levels of PPP1R11, phospho-Stat3 (S727), and the EMT marker Vimentin. In addition, culture at 0.5% O2 enhanced the levels of PPP1R11 and phospho-Stat3 (S727) in the organoids derived from the intestinal tumors of the compound mutant mice, but not in those from ApcMin/+ tumors.
To address whether the above-mentioned difference in the response to hypoxia between p53-deficient and p53-proficent CRC cells is determined by p53 status, the authors next tested the effect of p53 overexpression in p53-deficient SW480 cells. Ectopic expression of p53 abolished the enhancement of their invasion and migration activity induced by CoCl2, a chemical inducer of HIF1A, accompanied by reduced levels of PPP1R11 protein and phospho-STAT3 (S727). This repression of PPP1R11 by p53 was shown to be mediated by miR-34a. The authors further employed 3 isogenic CRC cell lines, namely HCT116, RKO, and SW48 cells, that differ only in TP53 status. In all three cell lines, TP53−/− cells showed a mesenchymal morphology, high levels of PPP1R11 and phospho-STAT3 (S727), and reduced level of miR-34a under hypoxia. In clear contrast, TP53+/+ cells under hypoxia did not adopt a mesenchymal morphology and showed upregulation of miR-34a and downregulation of PPP1R11. These results clearly indicate the essential roles of the TP53 status in determining the hypoxia responses of CRC cells.
Li et al. moved on to address the clinical relevance of their findings. They showed that while 5-FU treatment significantly reduced colony formation by HCT116 TP53+/+ cells under hypoxia (0.5% O2), 5-FU barely affected the colony forming activity of HCT116 TP53−/− cells at the same condition. Furthermore, knockdown of PPP1R11 abrogated the 5-FU resistance of HCT116 TP53−/− cells under hypoxia, connecting the p53/HIF1A/miR-34a/PPP1R11/STAT3 regulatory pathway to drug resistance of CRC. The authors finally evaluated their findings in clinical samples of CRC. Using the Cancer Genome Atlas (TCGA) database, they first showed that CRC samples with mutant TP53 displayed significantly higher expression of PPP1R11 mRNA compared with TP53-wild-type CRC samples. They then showed that the elevated PPP1R11 protein expression at invasion front of CRCs was significantly associated with liver metastasis. Indeed, the immunostaining pattern of PPP1R11 at invasion front overlapped with that of the EMT marker Laminin 52 and the hypoxia marker GLUT1, and the elevated expression of INH at the infiltrative tumor edge of CRCs was negatively correlated with the expression of miR-34a. They further demonstrated significant association between elevated expression of PPP1R11 and that of GLUT1 and Laminin 52 at the invasion front of primary tumors and metastases of CRCs.
As mentioned above, hypoxia has been implicated in invasion and metastasis of solid cancers through promotion of EMT, but hypoxia also induces p53. The meticulous and convincing study by Li et al. has unveiled the molecular mechanism by which the loss of p53 contributes to EMT and thereby invasion and metastasis of CRC through downregulation of miR-34a (Figure 1). Identification of PPP1R11 (INH3) as the key downstream effector that induces EMT via STAT3 phosphorylation provides a promising candidate target for therapy of invasive CRC containing hypoxic regions, in addition to the miR-34 mimics that are being developed (16,17). Like all excellent studies, the findings by Li et al. raise many questions. Is the p53/HIF1A/miR-34a/PPP1R11/STAT3 pathway involved in oncogenic signaling initiated by hypoxia-independent activation of HIF1A, such as the loss of VHL in kidney cancer? Is the axis also involved in the biology of cancer stem cells, which share many molecular characteristics with cancer cells undergoing EMT? CRC cells that have acquired EMT phenotypes in hypoxic environment will eventually invade into normoxic area to undergo intravasation. By citing their previous work (18), the authors state that it is conceivable that the transient repression of miR-34a by hypoxia observed here is fixated over time by DNA hypermethylation in its promoter region. Is such a fixation really taking place in CRC cells in vivo? Further studies are awaited to answer these and other remaining questions, and to develop effective means for preventing metastasis and/or circumventing drug resistance of CRCs.
Acknowledgments
Funding: This research is funded by grants from the Japan Society for the Promotion of Science (26290045).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Jinzhe Zhou (Department of General Surgery, Tongji Hospital, Tongji University School of Medicine, Shanghai, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ncri.2018.03.07). Dr. Aoki reports grants from The Hori Sciences & Arts Foundation, outside the submitted work. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nieto MA, Huang RY, Jackson RA, et al. EMT: 2016. Cell 2016;166:21-45. [Crossref] [PubMed]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15:178-96. [Crossref] [PubMed]
- Siemens H, Jackstadt R, Hünten S, et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle 2011;10:4256-71. [Crossref] [PubMed]
- Hahn S, Jackstadt R, Siemens H, et al. SNAIL and miR-34a feed-forward regulation of ZNF281/ZBP99 promotes epithelial-mesenchymal transition. EMBO J 2013;32:3079-95. [Crossref] [PubMed]
- Hur K, Toiyama Y, Takahashi M, et al. MicroRNA-200c modulates epithelial-tomesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 2013;62:1315-26. [Crossref] [PubMed]
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004;4:437-47. [Crossref] [PubMed]
- Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 2013;123:3664-71. [Crossref] [PubMed]
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009;139:871-90. [Crossref] [PubMed]
- Graeber TG, Osmanian C, Jacks T, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 1996;379:88-91. [Crossref] [PubMed]
- Li H, Rokavec M, Jiang L, et al. Antagonistic Effects of p53 and HIF1A on microRNA-34a Regulation of PPP1R11 and STAT3 and Hypoxia-induced Epithelial to Mesenchymal Transition in Colorectal Cancer Cells. Gastroenterology 2017;153:505-20. [Crossref] [PubMed]
- Hermeking H. MicroRNAs in the p53 network: Micromanagement of tumour suppression. Nat Rev Cancer 2012;12:613-26. [Crossref] [PubMed]
- Zhang J, Zhang L, Zhao S, et al. Identification and characterization of the human HCG V gene product as a novel inhibitor of protein phosphatase-1. Biochemistry 1998;37:16728-34. [Crossref] [PubMed]
- Haridas V, Nishimura G, Xu ZX, et al. Avicin D: a protein reactive plant isoprenoid dephosphorylates Stat 3 by regulating both kinase and phosphatase activities. PLoS One 2009;4:e5578 [Crossref] [PubMed]
- Zgheib C, Zouein FA, Chidiac R, et al. Calyculin A reveals serine/threonine phosphatase protein phosphatase 1 as a regulatory nodal point in canonical signal transducer and activator of transcription 3 signaling of human microvascular endothelial cells. J Interferon Cytokine Res 2012;32:87-94. [Crossref] [PubMed]
- Wen Z, Zhong Z, Darnell JE Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 1995;82:241-50. [Crossref] [PubMed]
- Wiggins JF, Ruffino L, Kelnar K, et al. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res 2010;70:5923-30. [Crossref] [PubMed]
- Bader AG. MiR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet 2012;3:120. [Crossref] [PubMed]
- Siemens H, Neumann J, Jackstadt R, et al. Detection of miR-34a promoter methylation in combination with elevated expression of c-Met and β-catenin predicts distant metastasis of colon cancer. Clin Cancer Res 2013;19:710-20. [Crossref] [PubMed]
Cite this article as: Aoki M. p53-regulated microRNA determines the fate of colorectal cancer cells under hypoxia. Non-coding RNA Investig 2018;2:22.


