
Placental miR-340 expression: a key to gestational programming of activity-based anorexia?
Anorexia nervosa (AN) is a severe eating disorder associated with the highest mortality rate among all mental disorders (1). However, despite its severity, the etiology of the disease is still to be better understood. Case-control studies indicate that women who experienced abuse in their childhood had a higher risk of developing an eating disorder later in life compared to controls without this life event (2). This association between early-life stress or trauma and psychiatric disorders is supported by a large cohort study (n=2,110,756) where prenatal and early-postnatal severe stress induced by the death of a close relative caused a higher risk of developing an eating disorder (3). It has been hypothesized that the placenta as key organ of maternal-fetal communication—besides its role to exchange nutrients and gases—mediates stress effects thereby influencing fetal neuronal development (4). Since trophoblast cells of the placenta originate from the embryo and therefore carry the sex of the fetus (XX or XY) (5), the placenta might contribute to sex-specific differences early on (6), possibly also in the development of AN known to affect females with a higher prevalence than males (1).
Further investigations have shown that numerous small non-coding RNAs, so called microRNAs (miRNA), are continuously released by trophoblasts of the placenta into the maternal blood (7). These miRNAs were originally found in the nematode Caenorhabditis elegans in 1993 (8) and regulate gene expression by inhibition of mRNA translation (9). A correlation between altered miRNA expression and anorexia has already been indicated in an anorexia mouse model showing that miRNA target genes were significantly upregulated in the hypothalamus and decreased in the cortex of anorectic mice (10).
Following up on these findings, Schroeder and colleagues further studied the etiology of AN investigating the origin of susceptibility to AN in utero by focusing on the placental miRNA expression using the activity-based anorexia (ABA) model (11).
Prenatal stress and ABA
ABA was established early on (12) and combines two core features of AN, namely food restriction and increased physical activity (13). Despite its limitations ABA is a well-established and so far best suited model to mimic AN in rodents.
When applying ABA to adolescent female mice, only 40% of the animals developed the ABA phenotype, while 60% were resistant to the protocol (11). In line with previous studies on hypothalamic dysregulation in AN (14,15), female ABA mice showed significant upregulation in gene expression for serotonin receptor (HTR) 1a (HTR1a) and hypocretin/orexin (Hcrt) and downregulation of agouti-related peptide (AgRP) and arginine vasopressin (AVP) compared to ABA resistant mice (11) giving rise to an involvement of these genes/gene products in the susceptibility to develop ABA.
Interestingly, prenatal stress caused ABA resistance in female mice as indicated by the fact that all males and prenatally stressed females scored significantly lower regarding ABA parameters (food intake, total activity, body weight change, circadian disruption, days until collapse, recovery of food intake) compared to female controls (11). Furthermore, prenatal stress induced elevated maternal corticosterone (CORT) levels in ABA mice, likely associated with downregulated placental 11ßhsd2 gene expression, an enzyme that inactivates CORT (11). These results extend clinical data that indicated an association between elevated maternal cortisol levels during the late second and third trimesters and an increased cortisol response after a painful heel-stick blood draw (16).
The role of placental miR-340 in gene regulation
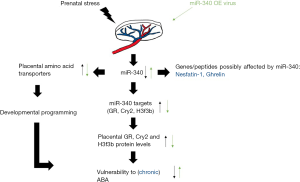
To further explore the effects of prenatal stress, several placental miRNAs were identified showing a pronounced downregulation of miR-340 following prenatal stress, where pregnant females were exposed to two stressors during the dark phase (e.g., multiple cage changes, immobilization for 30 min, swim stress for 15 min) and one further manipulation during the light phase (11). Located in the junctional zone of the placenta, miR-340 as part of the second intron of the Rnf130 gene is regulated by gene body methylation. Since gene body methylation normalizes gene overexpression, this mechanism might be used in the treatment of cancer (17).
Next, a higher methylation on the CpG2 sites of miR-340 was shown in the placenta of female prenatally stressed mice compared to placentas of non-stressed control females (11). Also, global DNA methylation was higher in females compared to males (11), indicating an impact of sex on the regulation of miR-340. This finding is in line with results of a previous genome study investigating the differences in methylation profiles in women with anorexia (n=29) and healthy controls (n=15) that showed elevated and less variable global DNA methylation patterns in women with AN (18).
Sex-dependent differences in ABA predisposition
The analysis of target genes for placental functioning in female mice that underwent prenatal stress indicated a significant upregulation of the glucocorticoid receptor (GR), cryptochrome 2 (Cyr2) and histone 3, family 3b (H3f3b) genes as well as their corresponding protein levels compared to controls (11). Interestingly, these gene and protein levels were inversely correlated with miR-340 expression levels (11). Glucocorticoids have been implicated in fetal development, especially in intrauterine growth or the risk of metabolic or neuroendocrine diseases later in life (19). It is to note that the fetal response to elevated glucocorticoid levels is sex dependent, resulting in hypertensive changes in females, while in males a glucocorticoid resistance has been observed (19).
When a female fetus shared space with only male in utero, miR-340 levels in the female fetus were significantly decreased compared to females accompanied by other females or females and males (11). This finding is likely associated with testosterone production shown to be produced by 18 days old male fetuses at high concentrations and released into the amniotic fluid (20). Taken together, this might indicate that prenatal androgen exposure and hence lower miR-340 levels on the one hand contribute to a decreased vulnerability towards ABA, and on the other hand give an explanation for the sex dependent differences in the incidence of AN.
The placenta as a gate to fetal developmental programming
One of the primary tasks of the placenta is to supply the fetus with nutrients, thereby allowing the offspring to grow and develop. Schroeder and colleagues investigated a possible correlation between placental miR-340 levels and the expression of placental nutrient transporters, focusing on the solute carrier (SLC) group, cholesterol transporters and the insulin-like growth factor (IGF) family. Igf2 is a fetal signal regulating placental growth and function whose deletion resulted in reduced placental growth, decreased passive permeability and ultimately fetal growth restriction (21). Schroeder and colleagues detected an inverse correlation of nutrient transporters and expression levels of miR-340 which was shown in the junctional zone of the placenta, where high miR-340 levels lead to a downregulation of Igf2 thereby reducing placental nutrient transport (11). This finding was further investigated in human placentas where also an inverse correlation of miR-340 levels and selective nutrient transporters was found (11). These data give rise to an impact of miR-340 on placental functioning in mouse as well as in human indicating that fetal nutrient acquisition is partly miR-340-dependent and can influence fetal development.
MiR-340 levels and vulnerability to ABA
These findings led to the assumption that overexpression of miR-340 might be associated with increased susceptibility to ABA. Transgenic mice of both sexes placenta-specifically overexpressing miR-340 induced by a lentivirus infecting the blastocysts’ trophoblast cells underwent the ABA protocol by the age of 30 days. Indeed, in female mice threefold overexpressing miR-340 73.3% developed ABA compared to only 36.4% mice developing ABA in the control virus group (11). Interestingly, mi-R340 overexpression also increased the susceptibility to ABA in males increasing the number of ABA-prone animals from 12.5% (control) to 50% (miR-340 overexpression) (11). Therefore, elevated miR-340 levels lead to an increase in ABA vulnerability in both sexes.
In summary, Schroeder and coworkers provide key experiments highlighting the role of miR-340 as a target substance in the prenatal programming of ABA (Figure 1). Since these data were generated under rather acute conditions, it will be interesting to investigate whether miR-340 is also altered in a more chronic model of ABA which was recently established in rats (22). In addition, it will be important to study whether these changes also impact on major food intake-regulatory hormones such as ghrelin (23) or nesfatin-1 (24) known to be affected under conditions of ABA. Lastly, the regulation of miR-340 will have to be investigated in patients with AN along with the characterization of the exact mechanisms how maternal stress leads to a downregulation of miR-340.
Acknowledgments
Funding: This work was supported by funding of the German Research Foundation (STE 1765/3-2) and Charité University Funding (UFF 89/441-176, A Stengel).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Jin Li (Cardiac Regeneration and Ageing Lab, School of Life Sciences, Shanghai University, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ncri.2018.09.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zipfel S, Giel KE, Bulik CM, et al. Anorexia nervosa: aetiology, assessment, and treatment. Lancet Psychiatry 2015;2:1099-111. [Crossref] [PubMed]
- Rayworth BB, Wise LA, Harlow BL. Childhood Abuse and Risk of Eating Disorders in Women. Epidemiology 2004;15:271-8. [Crossref] [PubMed]
- Su X, Liang H, Yuan W, et al. Prenatal and early life stress and risk of eating disorders in adolescent girls and young women. Eur Child Adolesc Psychiatry 2016;25:1245-53. [Crossref] [PubMed]
- Bronson SL, Bale TL. The Placenta as a Mediator of Stress Effects on Neurodevelopmental Reprogramming. Neuropsychopharmacology 2016;41:207-18. [Crossref] [PubMed]
- Nugent BM, Bale TL. The omniscient placenta: Metabolic and epigenetic regulation of fetal programming. Front Neuroendocrinol 2015;39:28-37. [Crossref] [PubMed]
- Clifton VL. Review: Sex and the Human Placenta: Mediating Differential Strategies of Fetal Growth and Survival. Placenta 2010;31:S33-9. [Crossref] [PubMed]
- Luo SS, Ishibashi O, Ishikawa G, et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod 2009;81:717-29. [Crossref] [PubMed]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993;75:843-54. [Crossref] [PubMed]
- Djuranovic S, Nahvi A, Green R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science 2012;336:237-40. [Crossref] [PubMed]
- Mercader JM, Gonzalez JR, Lozano JJ, et al. Aberrant brain microRNA target and miRISC gene expression in the anx/anx anorexia mouse model. Gene 2012;497:181-90. [Crossref] [PubMed]
- Schroeder M, Jakovcevski M, Polacheck T, et al. Placental miR-340 mediates vulnerability to activity based anorexia in mice. Nat Commun 2018;9:1596. [Crossref] [PubMed]
- Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol 1967;64:414-21. [Crossref] [PubMed]
- Scharner S, Prinz P, Goebel-Stengel M, et al. Activity-Based Anorexia Reduces Body Weight without Inducing a Separate Food Intake Microstructure or Activity Phenotype in Female Rats-Mediation via an Activation of Distinct Brain Nuclei. Front Neurosci 2016;10:475. [Crossref] [PubMed]
- Vink T, Hinney A, van Elburg AA, et al. Association between an agouti-related protein gene polymorphism and anorexia nervosa. Mol Psychiatry 2001;6:325-8. [Crossref] [PubMed]
- Nilsson IA, Lindfors C, Schalling M, et al. Anorexia and hypothalamic degeneration. Vitam Horm 2013;92:27-60. [Crossref] [PubMed]
- Davis EP, Glynn LM, Waffarn F, et al. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry 2011;52:119-29. [Crossref] [PubMed]
- Yang X, Han H, De Carvalho DD, et al. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell 2014;26:577-90. [Crossref] [PubMed]
- Booij L, Casey KF, Antunes JM, et al. DNA methylation in individuals with anorexia nervosa and in matched normal-eater controls: A genome-wide study. Int J Eat Disord 2015;48:874-82. [Crossref] [PubMed]
- Bivol S, Owen SJ, Rose'Meyer RB. Glucocorticoid-induced changes in glucocorticoid receptor mRNA and protein expression in the human placenta as a potential factor for altering fetal growth and development. Reprod Fertil Dev 2016;29:845-54. [Crossref] [PubMed]
- vom Saal FS, Quadagno DM, Even MD, et al. Paradoxical effects of maternal stress on fetal steroids and postnatal reproductive traits in female mice from different intrauterine positions. Biol Reprod 1990;43:751-61. [Crossref] [PubMed]
- Constancia M, Hemberger M, Hughes J, et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 2002;417:945-8. [Crossref] [PubMed]
- Frintrop L, Trinh S, Liesbrock J, et al. Establishment of a chronic activity-based anorexia rat model. J Neurosci Methods 2018;293:191-8. [Crossref] [PubMed]
- Francois M, Barde S, Achamrah N, et al. The number of preproghrelin mRNA expressing cells is increased in mice with activity-based anorexia. Neuropeptides 2015;51:17-23. [Crossref] [PubMed]
- Scharner S, Prinz P, Goebel-Stengel M, et al. Activity-based anorexia activates nesfatin-1 immunoreactive neurons in distinct brain nuclei of female rats. Brain Res 2017;1677:33-46. [Crossref] [PubMed]
Cite this article as: Kühne SG, Stengel A. Placental miR-340 expression: a key to gestational programming of activity-based anorexia? Non-coding RNA Investig 2018;2:53.


