
Role for miR-29b suppression in multiple myeloma
Multiple myeloma (MM) is a common hematologic malignancy of the elderly, accounting for 2% of cancer deaths, in which malignant plasma cells proliferate in the bone marrow resulting in bone disease and immunosuppression (1,2). Antigen-presenting dendritic cells (DCs) regulate activation of the immune response and tolerance by inducing differentiation of T-helper lymphocytes into functional T cell populations (3). DCs play a key role in reprogramming to sustain MM and are targets for therapeutic intervention (4). Non-coding RNAs including miRNAs and lncRNAs are dysregulated in MM cell lines, serum of MM patients, and peripheral mononuclear cells [reviewed in (5,6)]. A recent report: “MiR-29b antagonizes the pro-inflammatory tumor-promoting activity of multiple myeloma-educated dendritic cells” by Botta et al. (7) provides evidence for DC-MM cross-talk involving reduced miR-29b expression in DCs. The authors demonstrated that miR-29b was uniquely downregulated in MM tumor-associated DCs (TA-DCs) in microarray studies in mouse and human samples.
There are two chromosomal locations of miR-29b in the human genome: miR-29b-2 on chromosome 1q32.2 and miR-29b-1 on chromosome 7q32.3. Initial reports suggested that miR-29b-1 preferentially localizes to the nucleus (8,9), but more recent single-molecule fluorescence-based analysis revealed that although miR-29b localizes to the nucleus of human cells, it does not do so more than other miRNAs (10). The transcription of both miR-29b-1 and miR-29b-2 is repressed by MYC and NFκB (9) while STAT3, YY1, and JUN inhibit and SP1 stimulates miR-29b-1 transcription (11). The authors of this report (7) did not distinguish whether both chromosomal locations were the source of miR-29b in DCs, or if one locus was active, nor did they examine the expression of miR-29a or miR-29c which are co-transcribed in the primary transcript (pri-miR) of miR-29b-1 and miR29-b2, respectively (12). Moreover, they did not investigate the mechanism of miR-29b downregulation in DCs associated with MM. In earlier work, the investigators had reported downregulation of miR-29b in MM cell lines and showed that overexpression of miR-29b in MM cells inhibited cell growth and stimulated apoptosis in vitro and as xenograft tumors in mice (13). They have also characterized miR-29b targets in MM including SP1 (13) and have reviewed the “epi-miR” activity of miR-29b in regulating epigenetic changes by downregulating DNMT3A and DNMT3B (11). Others reported that NOD2, a cytoplasmic pattern recognition receptor, is required for the induction of all miR-29 family members in human DCs (14). As in the Botta et al. study (7), the Brain et al. group identified multiple inflammatory genes regulated by loss of miR-29 expression in DCs in Crohn’s disease, and identified IL12B (IL-12p40) as a direct target of miR-29 via its 3'UTR (14). They also demonstrated that miR-29−/− mice showed worse DSS-induced colitis than wildtype mice which was associated with increased IL-23 and Th17-signature cytokines (14), results parallel to those in the Botta et al. report (7).
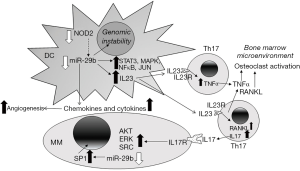
The investigators concluded that reduction of miR-29b in MM-TA-DCs resulted in a loss of repression of immune-related, pro-inflammatory signaling molecules and cytokines, including IL12B, NKFB1, MAP2K4, SP1, CCL2, CXCL2 (MIP2a), CXCL8 (IL8), CCL8 (MCP2), CXCL12, CCL7 (MCP3), CXCL5, IL10, CXCL10 (IP10), CXCL16, MCP1, IL23A. Results of were confirmed by miR-29b mimic transfection of DCs co-cultured with MM cells, showing that miR-29b mimics reduced pro-inflammatory networks in DCs. The authors state that this is the first published demonstration that overexpression of a miRNA in DCs produced changes in co-cultured MMs. Examination of clinical data from MM patients for chemokine/cytokine receptor expression showed that patients with high CCR3 and CXCR3 had a “worse outcome” while high CCR4 and CXCR6 expression correlated with short progression free survival. These data complement the in vitro DC-MM studies. The authors also demonstrated that PTEN is a direct target of miR-29b using a 3'UTR-luciferase reporter assay in miR-29b-mimic expressing DCs. PTEN was previously demonstrated to be directly targeted by miR-29a interaction in the 3'UTR (15). Overexpression of miR-29b in the DCs-co-cultured with MMs inhibited MM cell growth and motility and decreased phosphorylation of ERK, AKT, and SRC, commensurate with PTEN suppression. Likewise, miR-29b-mimic overexpression in DC cells and the DC-MM co-culture produced anti-angiogenic molecules in the conditioned medium that repressed the induction of capillary-like structures in human umbilical vein endothelial cells, supporting a role for miR-29b as anti-angiogenic under non-malignant conditions in bone marrow. They did not examine whether exosomes produced by DCs could be involved in the transmission of materials, including miRNAs, between DCs and MMs and produce the reported effects. DCs co-cultured with MM cells stimulated Th17 lymphocyte polarization in an IL23A-dependent manner. In NOD-SCID-synth-hu mice with MM engraftment in bio-synthetic polymer scaffolds that were injected with miR-29b mimics, MM cell proliferation, CD31+ vessel staining, and DC staining for IL-23 were reduced, suggesting that miR-29b mimics reduce IL-23 secretion, angiogenesis, and MM proliferation in vivo. Lymphocyte-DC co-culture experiments showed that miR-29b mimic-overexpression in DCs reduced the population of Th17 CD4/CD161 double positive cells, indicating that miR-29b inhibits Th17 polarization, a finding that has therapeutic implications. Finally, miR-29b-overexpressing DCs reduced genomic instability in co-cultured MMs as shown by lower ATM and ATR phosphorylation, hallmarks of the DNA damage response [reviewed in (16)]. The authors conclude that deregulation of miR-29b in CDs and MM cells plays an important role in MM pathogenesis and that miR-29b-based therapeutics are of keen interest for development to target MM.
In summary, the paper by Botta et al. demonstrate that miR-29b plays a role in normal DC function that is abrogated in MM (7). The consequences of decreased miR-29b in DCs (7) and MM (13) is diagramed in Figure 1 which also includes established release of TNFα and RANKL from activated Th17 (17). Future studies addressing the mechanism of miR-29b loss and targeting its upregulation or localized overexpression within the bone marrow may be useful in treating MM.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Rongrong Gao (Department of Cardiology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ncri.2018.06.02). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vallet S, Pecherstorfer M, Podar K. Adoptive cell therapy in multiple Myeloma. Expert Opin Biol Ther 2017;17:1511-22. [Crossref] [PubMed]
- Zweegman S, Palumbo A, Bringhen S, et al. Age and aging in blood disorders: multiple myeloma. Haematologica 2014;99:1133-7. [Crossref] [PubMed]
- Shissler SC, Lee MS, Webb TJ. Mixed Signals: Co-Stimulation in Invariant Natural Killer T Cell-Mediated Cancer Immunotherapy. Front Immunol 2017;8:1447. [Crossref] [PubMed]
- Weinstock M, Rosenblatt J, Avigan D. Dendritic Cell Therapies for Hematologic Malignancies. Mol Ther Methods Clin Dev 2017;5:66-75. [Crossref] [PubMed]
- Zhu B, Ju S, Chu H, et al. The potential function of microRNAs as biomarkers and therapeutic targets in multiple myeloma. Oncol Lett 2018;15:6094-106. [PubMed]
- Nobili L, Ronchetti D, Agnelli L, et al. Long Non-Coding RNAs in Multiple Myeloma. Genes (Basel) 2018;9: [Crossref] [PubMed]
- Botta C, Cucè M, Pitari MR, et al. MiR-29b antagonizes the pro-inflammatory tumor-promoting activity of multiple myeloma-educated dendritic cells. Leukemia 2018;32:1003-15. [Crossref] [PubMed]
- Hwang HW, Wentzel EA, Mendell JT. A hexanucleotide element directs microRNA nuclear import. Science 2007;315:97-100. [Crossref] [PubMed]
- Kriegel AJ, Liu Y, Fang Y, et al. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics 2012;44:237-44. [Crossref] [PubMed]
- Pitchiaya S, Heinicke LA, Park JI, et al. Resolving Subcellular miRNA Trafficking and Turnover at Single-Molecule Resolution. Cell Rep 2017;19:630-42. [Crossref] [PubMed]
- Amodio N, Rossi M, Raimondi L, et al. miR-29s: a family of epi-miRNAs with therapeutic implications in hematologic malignancies. Oncotarget 2015;6:12837-61. [Crossref] [PubMed]
- Schmitt MJ, Margue C, Behrmann I, et al. MiRNA-29: a microRNA family with tumor-suppressing and immune-modulating properties. Curr Mol Med 2013;13:572-85. [Crossref] [PubMed]
- Amodio N, Di Martino MT, Foresta U, et al. miR-29b sensitizes multiple myeloma cells to bortezomib-induced apoptosis through the activation of a feedback loop with the transcription factor Sp1. Cell Death Dis 2012;3:e436 [Crossref] [PubMed]
- Brain O, Owens BM, Pichulik T, et al. The intracellular sensor NOD2 induces microRNA-29 expression in human dendritic cells to limit IL-23 release. Immunity 2013;39:521-36. [Crossref] [PubMed]
- Kong G, Zhang J, Zhang S, et al. Upregulated microRNA-29a by hepatitis B virus X protein enhances hepatoma cell migration by targeting PTEN in cell culture model. PLoS One 2011;6:e19518 [Crossref] [PubMed]
- Blackford AN, Jackson SP. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol Cell 2017;66:801-17. [Crossref] [PubMed]
- Yago T, Nanke Y, Kawamoto M, et al. IL-23 and Th17 Disease in Inflammatory Arthritis. J Clin Med 2017;6: [Crossref] [PubMed]
Cite this article as: Klinge CM. Role for miR-29b suppression in multiple myeloma. Non-coding RNA Investig 2018;2:36.


