
A circular RNA regulator quaking: a novel gold mine to be unfolded in doxorubicin-mediated cardiotoxicity
The death rate from cancer in the USA has declined steadily over the past 2 decades (1). Improved cancer survival associated with anticancer agents has led to a greater recognition of the late effects of cardiotoxicity including left ventricular dysfunction, heart failure and coronary heart disease. Anthracyclines (doxorubicin, daunorubicin, epirubicin and idarubicin) are used in a wide range of malignancies including breast cancer and lymphoma. Anthracycline administration is associated with dose-related cardiomyocyte injury and death, leading to heart failure. The main mechanism of anthracycline cardiotoxicity is thought to be by inhibition of topoisomerase 2β, resulting in activation of cell death pathways and inhibition of mitochondrial biogenesis (2).
Transcribed RNAs can be grouped into protein-coding messenger RNAs (mRNAs) and noncoding RNAs (ncRNAs). NcRNAs are regions of the genome that do not code for proteins, but can regulate the function of genes and thus modulate a vast number of physiological and pathological processes. Circular RNAs (circRNAs) are one family of the long ncRNAs, which are ubiquitously expressed in mammalian tissues and important gene regulators by diverse mechanisms. It has been increasingly clear that numerous circRNAs are formed by back splicing of exons, suggesting previously unrecognized regulatory mechanisms of coding sequences (3). As to the global mechanisms of circRNA function, some circRNAs contain binding sites for microRNAs (miRNAs), acting as miRNA sponges, while others possess binding sites for proteins and function as protein sponges. It is known that a few circRNAs in the nucleus regulate the transcription of host genes. Lastly, some circRNAs can even code for proteins in a 5’ cap-independent manner (4). Among highly abundant human cardiac-specific circRNAs, more than 100 circRNA isoforms are generated from Titin (Ttn) and Ryanodine receptor 2 (RYR2), and they are suggested to have important roles in regulation of cardiac genes (5). The functional roles of circRNAs in cardiovascular diseases (CVDs) such as myocardial infarction, ischemia-reperfusion injury, atherosclerosis, cardiomyopathy and cardiac fibrosis have been increasingly reported. Multiple databases have also provided excellent platforms to facilitate further functional research on circRNAs and to obtain first insights into roles of identified cardiac circRNAs (4).
The processing and function of both coding and ncRNAs are dependent on RNA-binding proteins (RBPs). RBPs are rapidly emerging as pivotal players in various pathophysiological processes because they are involved in the regulation of global RNA processing and gene expression. For example, RBPs mediate post-transcriptional events that significantly impact pre-mRNA fate such as alternative splicing, RNA stability, subcellular localization, and ribosome-mediated translation of mature mRNAs (6). Many interactions between RBPs and RNAs are specific and require a mechanism for recognition of the appropriate binding target. When an RBP’s function depends on recognizing a specific RNA sequence or secondary/tertiary structure, it is possible to learn the determinants of the interaction. Indeed, the development of models for RBP specificity has become an active area of bioinformatics research. Important new high-throughput methods and data resources, which are used to model RBP-RNA interactions, have been also introduced (7).
Quaking (QKI) is an RBP belonging to the Signal Transduction and Activation of RNA (STAR) family (8). QKI has been shown to regulate pre-mRNA splicing, mRNA turnover and translation, and has been implicated in the regulation of epithelial-to-mesenchymal transition via its effects on circular RNA formation (9). Qki gene encodes alternatively spliced QKI isoforms that differ in their C-terminal 8-30 amino acid sequences. Cytoplasmic QKI-7 was shown to induce apoptosis in fibroblasts and primary rat oligodendrocytes. The co-expression of either QKI-5 or QKI-6 with QKI-7 caused the nuclear translocation of QKI-7, thus suppressing QKI-7-mediated apoptosis (10). QKI-5 is mostly studied in lung cancer cells and a previous study showed that its downregulation leads to increased cell proliferation (11). Another recent study revealed that QKI-5 inhibits pro-metastasis processes of lung cancer cells by inhibiting β-catenin signaling pathway, and that hypermethylation of QKI-5 promoter is a crucial epigenetic mechanism of reducing QKI-5 expression in lung cancer cells (12).
In a recent issue of Circulation Research, Gupta et al. (13) investigated the role of QKI in doxorubicin-mediated cardiotoxicity via regulating cardiac circRNAs. First, the authors performed global transcriptome profiling analyses on cardiac tissues of doxorubicin-treated mice to identify RBPs involved in doxorubicin-induced cardiotoxicity and used published RBPDB, the database of RBP specificities (14). Their further analyses identified five dysregulated RBPs, and they found that only one RBP, QKI was downregulated in response to doxorubicin. Qki has been reported to inhibit cardiac apoptosis during ischemia-reperfusion injury by regulating the stability of Foxo1 mRNA (15). Interestingly, Qki5 is found to be the most abundant isoform in the heart with the highest expression in cardiomyocytes. Gupta et al. (13) further demonstrated that QKI levels were significantly downregulated in doxorubicin-treated HL-1 cardiomyocytes, primary rat cardiomyocytes and human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs). To explore a potential functional role of Qki in cardiomyocytes, the authors performed loss- and gain-of-function experiments using siRNA-mediated Qki5 knockdown in primary rat cardiomyocytes and lentiviral-mediated stable overexpression in HL-1 cell lines, and demonstrated that Qki5 protects doxorubicin-mediated cardiomyocyte apoptosis and atrophy (13). As Qki was previously reported to regulate the formation of circRNAs during epithelial-to-mesenchymal transition (9), Gupta et al. (13) selected a panel of highly cardiac-enriched circRNAs, which are derived from previously published RNA-Sequencing datasets (16), to screen circRNAs regulated by Qki in the heart. CircRNAs derived from the genes Ttn, Formin homology 2 domain containing 3 (Fhod3), and Striatin, calmodulin-binding protein 3 (Strn3) were only validated to be positively regulated with Qki expression and significantly downregulated in doxorubicin-treated mouse hearts. Their additional data suggest that circRNAs, which are especially derived from Ttn, are downstream mediators of Qki5-mediated cardioprotective effects. The authors also conducted adeno-associated virus 9 (AAV9)-mediated overexpression of QKI-5 in the mouse heart to investigate whether in vivo overexpression of QKI-5 also reduces doxorubicin-mediated cardiotoxicity. However, in contrary to the in vitro results, too high overexpression of Qki5 with 2×1012 of AAV9 exacerbated doxorubicin-mediated cardiotoxicity in mice. The authors postulated that cytoplasmic accumulation of Qki5 due to excess overexpression contributed to these contradicting results, because QKI-5 has been shown to have nuclear localization signal and mediate nuclear translocation of other isoforms by hetero-dimerization (10). However, Gupta et al. (13) also showed that a modest overexpression of Qki5 with 7.5×1011 of AAV9 in the mouse model resulted in preserved nuclear localization and attenuated doxorubicin-induced cardiotoxicity. Based on their in vivo findings, the authors hypothesized that too high expression of QKI-5 favors homo-dimerization, not hetero-dimerization with other isoforms, thus leaving QKI-6 and QKI-7 to stay in cytoplasm to initiate apoptosis. In support of this notion, another previous study demonstrated that QKI-7 can induce apoptosis in fibroblasts and primary rat oligodendrocytes, and that a balance between the alternatively spliced QKI isoforms is required for cell survival (10), suggesting that too much overexpression of QKI-5 might cause imbalance with the other QKI isoforms and lead to apoptosis and cardiac dysfunction (Figure 1A).
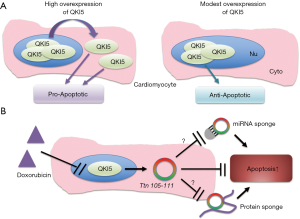
Using elegant studies, Gupta et al. (13) discovered Qki5 as a new target molecule to alleviate doxorubicin-induced cardiotoxic effects through regulation of cardiac circRNAs. The cardioprotective effect of QKI is supported by a previous study, reporting that QKI inhibits ischemia/reperfusion-induced cardiomyocyte apoptosis (15). Because some circRNAs are known to bind to miRNAs and act as miRNA sponges, as well as QKI-5 and QKI-6 isoforms regulate glial cell function by modulating the processing of miRNA-7 (17), QKI-5 might also regulate some miRNAs in the heart in response to doxorubicin through activation of circRNAs that Gupta et al. (13) identified (Figure 1B). Although Gupta et al. (13) especially focused on circRNA Ttn 105-111 as a downstream mediator of QKI-5, it is of interest to elucidate the roles of the other circRNA candidates such as Fhod3 and Strn3 2-7 in doxorubicin-mediated heart failure.
As to the functional relationship between RBPs and circRNAs, Khan et al. (18) recently also reported that RNA-binding motif protein 20 (RBM20) regulates circRNA production from the Ttn gene. Interestingly, their transcriptome analyses of circRNAs in human hearts revealed that circRNAs from Ttn gene are dysregulated in cardiomyopathy (mainly in dilated cardiomyopathy, DCM). Non-sense or frameshift mutations in the A-band domain of Ttn, which lead to truncated Ttn proteins, have been implicated as a major cause for DCM (19,20). Interestingly, Khan et al. (18) also found that RBM20 is crucial for the formation of a subset of circRNAs that are originated from a specific region within the I-band of Ttn. This region is known to undergo extensive alternative splicing to produce Ttn isoforms. In a similar fashion, the further study, which investigates how QKI is required for the formation of circRNAs, would be beneficial to delineate an underlying mechanism of their action, and establish the functional relationship between QKI and circRNA Ttn 105-111.
Although Gupta et al. (13) claimed that circRNA Ttn 105-111 is a downstream mediator of Qki5-mediated protective effects on doxorubicin-induced cardiomyocytes, underlying mechanisms by which circRNA Ttn 105-111 inhibits cardiomyocyte apoptosis remain to be determined. Moreover, it is unclear how this circRNA is regulated in cardiomyocytes when overexpressed QKI-5 is located in cytoplasm and leads to cellular apoptosis and atrophy. Further studies using loss- or gain-of-function mouse models of circRNAs from Ttn gene are warranted to explore whether these circRNAs confer cardioprotective effects against doxorubicin-induced cardiotoxicity. Interestingly, Li et al. (21) showed that Qki null embryos died between embryonic day 9.5 (E9.5) and E10.5 due to the failure of blood circulation in the yolk sac. Qki is also expressed in endothelial and smooth muscle cells, and Qki regulates vascular remodeling and angiogenesis. Although Gupta et al. (13) demonstrated that QKI in cardiomyocytes regulates apoptosis and atrophy, QKI in endothelial and smooth muscle cells may also play a role in doxorubicin-induced cardiomyopathy.
Gupta et al. (13) also demonstrated that QKI mRNA was significantly downregulated in patients with ischemic or dilated cardiomyopathy compared to healthy controls, suggesting that QKI may be downregulated in other heart failure models. In order to clarify whether the cardioprotective effect of QKI-5 is specific in doxorubicin-induced cardiotoxicity or not, additional studies should be conducted using other mouse models of heart failure such as myocardial infarction or transverse aortic constriction. Of note, as the authors described, in vivo mild overexpression of Qki5 led to not only inhibition of cardiomyocyte apoptosis in the presence of doxorubicin but also cardiac hypertrophy and increased left ventricular ejection fraction in the basal condition. Considering the therapeutic potential for doxorubicin-mediated cardiotoxicity in the future, it would be incredibly important to clarify the mechanism by which QKI-5 regulates cardiac hypertrophy.
Taken together, Gupta et al. (13) provides new insights into the function of QKI-5 in doxorubicin-induced cardiotoxicity via regulation of cardiac circRNAs and suggests QKI-5 as a therapeutic target with a narrow window. However, there are several caveats to be elucidated: (I) the detailed roles of candidate QKI-5-regulatable circRNAs, especially Ttn 105-111, in doxorubicin-induced cardiomyopathy are still unknown, and the functional relationship between these circRNAs and QKI-5 has not been established; (II) in vivo mild overexpression of QKI-5 improves cardiac function, which is not with higher overexpression of QKI-5. This makes one to question its therapeutic window and clinical efficacy; (III) cardiac function is also improved with low dose of QKI-5 without doxorubicin, suggesting its direct role in contractility, not in doxorubicin-induced cardiotoxicity. Although future studies using gain- or loss-of-function of QKI-5-regulatable circRNAs along with the other heart failure models are warranted to address the aforementioned limitations, the establishment of an RBP-circRNA axis in doxorubicin-mediated cardiotoxicity, as outlined in the work by Gupta et al. (13), may pave the way for new therapeutic strategies for cardiac pathophysiology.
Acknowledgments
We thank the editors for inviting us to write this editorial.
Funding: This work was supported by National Institutes of Health R01 [HL134354 and AR070029 to Y Tang, and HL124251 to IM Kim]; American Physiological Society Shih-Chun Wang Young Investigator Award [APHYS00008 to IM Kim]; and American Heart Association [Grant-in-Aid 12GRNT12100048 and Scientist Development Grant 14SDG18970040 to IM Kim].
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shengguang Ding (The Second Affiliated Hospital of Nantong University, Nantong, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ncri.2018.04.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013;495:333-8. [Crossref] [PubMed]
- Bayoumi AS, Aonuma T, Teoh JP, et al. Circular noncoding RNAs as Potential Therapies and Circulating Biomarkers for Cardiovascular Diseases. Acta Pharmacologica Sinica 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Tan WL, Lim BT, Anene-Nzelu CG, et al. A landscape of circular RNA expression in the human heart. Cardiovasc Res 2017;113:298-309. [PubMed]
- de Bruin RG, Rabelink TJ, van Zonneveld AJ, et al. Emerging roles for RNA-binding proteins as effectors and regulators of cardiovascular disease. Eur Heart J 2017;38:1380-8. [PubMed]
- Re A, Joshi T, Kulberkyte E, et al. RNA-protein interactions: an overview. Methods Mol Biol 2014;1097:491-521. [Crossref] [PubMed]
- Galarneau A, Richard S. Target RNA motif and target mRNAs of the Quaking STAR protein. Nat Struct Mol Biol 2005;12:691-8. [Crossref] [PubMed]
- Conn SJ, Pillman KA, Toubia J, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell 2015;160:1125-34. [Crossref] [PubMed]
- Pilotte J, Larocque D, Richard S. Nuclear translocation controlled by alternatively spliced isoforms inactivates the QUAKING apoptotic inducer. Genes Dev 2001;15:845-58. [Crossref] [PubMed]
- Zong FY, Fu X, Wei WJ, et al. The RNA-binding protein QKI suppresses cancer-associated aberrant splicing. PLoS Genet 2014;10:e1004289 [Crossref] [PubMed]
- Zhou X, Li X, Sun C, et al. Quaking-5 suppresses aggressiveness of lung cancer cells through inhibiting beta-catenin signaling pathway. Oncotarget 2017;8:82174-84. [PubMed]
- Gupta SK, Garg A, Bar C, et al. Quaking Inhibits Doxorubicin-Mediated Cardiotoxicity Through Regulation of Cardiac Circular RNA Expression. Circ Res 2018;122:246-54. [Crossref] [PubMed]
- Cook KB, Kazan H, Zuberi K, et al. RBPDB: a database of RNA-binding specificities. Nucleic Acids Res 2011;39:D301-8. [Crossref] [PubMed]
- Guo W, Jiang T, Lian C, et al. QKI deficiency promotes FoxO1 mediated nitrosative stress and endoplasmic reticulum stress contributing to increased vulnerability to ischemic injury in diabetic heart. J Mol Cell Cardiol 2014;75:131-40. [Crossref] [PubMed]
- Werfel S, Nothjunge S, Schwarzmayr T, et al. Characterization of circular RNAs in human, mouse and rat hearts. J Mol Cell Cardiol 2016;98:103-7. [Crossref] [PubMed]
- Wang Y, Vogel G, Yu Z, et al. The QKI-5 and QKI-6 RNA binding proteins regulate the expression of microRNA 7 in glial cells. Mol Cell Biol 2013;33:1233-43. [Crossref] [PubMed]
- Khan MA, Reckman YJ, Aufiero S, et al. RBM20 Regulates Circular RNA Production From the Titin Gene. Circ Res 2016;119:996-1003. [PubMed]
- Roberts AM, Ware JS, Herman DS, et al. Integrated allelic, transcriptional, and phenomic dissection of the cardiac effects of titin truncations in health and disease. Sci Transl Med 2015;7:270ra6 [Crossref] [PubMed]
- Herman DS, Lam L, Taylor MR, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med 2012;366:619-28. [Crossref] [PubMed]
- Li Z, Takakura N, Oike Y, et al. Defective smooth muscle development in qkI-deficient mice. Dev Growth Differ 2003;45:449-62. [Crossref] [PubMed]
Cite this article as: Aonuma T, Bayoumi AS, Tang Y, Kim IM. A circular RNA regulator quaking: a novel gold mine to be unfolded in doxorubicin-mediated cardiotoxicity. Non-coding RNA Investig 2018;2:19.


