
miRNAs regulate development and function of regulatory T-cells in recent onset islet autoimmunity in pre-Type 1 diabetes
Circulating miRNAs in Type 1 diabetes (T1D)
T1D is an autoimmune disorder resulting from selective destruction of pancreatic β-cells by the immune system. Abnormal immune responses triggered by environmental factors result in pancreatic β-cell loss and insulin deficiency in genetically susceptible individuals (1). Non-coding RNAs including microRNAs (miRNAs) have been shown be important participants in T1D pathogenesis. miRNAs are short (~22 nucleotides) non-coding RNAs that regulate gene expression in a posttranscriptional manner (2). In general, the miRNAs exert their functions via binding with the 3' untranslated regions (UTRs) of their target genes, resulting in translational inhibition or direct degradation of the targeted mRNA leading to diminished protein expression (2,3). Alterations in miRNA expression have been associated with several human autoimmune and inflammatory diseases including T1D (4,5).
A growing number of studies have shown that circulating miRNAs play important roles in T1D immunopathogenesis by modulating immune cell differentiation, development, activation and function (6,7). Several studies have generated circulating miRNA profiles from serum, plasma and blood cells in newly diagnosed T1D patients and investigated their role as potential diagnostic biomarkers to predict disease progression (8-10). Sebastiani et al. and Nielsen et al. were one of the first to compare whole blood and serum miRNA profiles from newly diagnosed T1D patients and healthy controls and found differentially expressed miRNAs in T1D patients that regulate the function of immune cells and/or pancreatic β-cells (8,9). A highly upregulated miRNA miR‐25 predicted residual β‐cell function (C‐peptide) and glycaemic control (HbA1c) 3 months after disease onset (9). Expression levels of miR‐21a and miR‐93 were downregulated in peripheral blood mononuclear cells (PBMC) of T1D patients compared with healthy controls (10). Both miRNAs participate in NF-κB signalling pathway and negatively regulate key apoptotic and inflammatory genes. Recently, a systematic review and meta-analysis of studies comparing miRNA expression profiles from T1D patients and non-diabetic controls in T1D related tissues reported 11 circulating miRNAs as the most promising potential biomarkers of T1D (11). These eleven miRNAs (miR-21-5p, miR-24-3p, miR-100-5p, miR-146a-5p, miR-148a-3p, miR-150-5p, miR-181a-5p, miR-210-5p, miR-342-3p, miR-375 and miR-1275) were involved in pathways related to immune system, cell proliferation, insulin biosynthesis and secretion and might play significant roles in autoimmune responses in T1D.
miRNAs and islet-autoimmunity
The onset of islet autoimmunity in children (pre-T1D) is indicated by development of multiple autoantibodies reacting with autoantigens like insulin, glutamic acid decarboxylase (GAD), islet antigen 2 (IA-2) and islet zinc transporter. Increasing evidence shows that circulating miRNAs associate with ongoing islet autoimmunity in T1D patients (12-15). Expression of miR-146a, one of the most downregulated miRNA in PBMCs from newly diagnosed T1D patients associated with high serum GAD autoantibody titers (12). miR-326, a highly expressed miRNA in peripheral blood lymphocytes was further upregulated in T1D patients positive for islet autoantibodies against GAD and IA-2 (13). In a large cohort of high-risk autoantibody-positive relatives of T1D patients matched with autoantibody-negative, family matched siblings, serum levels of certain miRNAs reflected islet autoimmunity and its progression (14). Specifically, miR-21-3p, miR-29a-3p and miR-424-5p showed most robust associations with increased risk of disease progression in high-risk relatives with multiple autoantibodies (14). Very few studies have focused on associations between ongoing islet autoimmunity and miRNA levels during the pre-diabetic period. A recent report by Åkerman et al. found no significant differences between serum miRNA profiles from high-risk individuals (positive for multiple islet autoantibodies) compared to non-progressors (15). However, the miRNA expression from high risk group significantly correlated with glycemic status and ongoing islet autoimmunity (15).
miRNA mediated T-cell dysfunction in recent onset islet autoimmunity
A number of miRNAs play crucial roles in regulation of T cell development and function (16). Several factors including changes in miRNA expression, pro-inflammatory cytokines, and reduced Forkhead box protein 3 (FOXP3) activity contributes to impaired regulatory T cell function and accelerated islet autoimmunity during pre-T1D. Dysregulated miRNAs in CD4+ T cell subsets including natural regulatory CD4+ FOXP3+ T cells (Tregs) from high-risk individuals have been suggested as markers of increased risk and T cell dysfunction in T1D (7,17-19). Zhang et al. observed distinct miRNA expression patterns between specific regulatory T cell subsets in high risk first-degree relatives of T1D patients positive for multiple autoantibodies (pre-T1D) compared to healthy controls (18). Influence of T cell activation on miRNA profiles was investigated by in vitro activation of naïve Treg cells followed by small RNA-sequencing of CD4+ T cell subsets. Multiple miRNAs were found differentially expressed in naïve CD4+ T cells and activated Treg cells of pre-T1D individuals compared to healthy controls. In contrast, no significant differences in miRNA expression were observed in naïve Treg cells between the two groups (18). These findings indicate that miRNA profiles of activated Treg cells are predictors of impaired Treg function in pre-T1D.
Recently, Serr et al. observed reduced Treg induction in CD4+ T cells from recent onset islet autoimmunity (<5 years autoantibody positive) children compared to autoantibody negative and long term autoimmunity (>10 year autoantibody positive) children without clinical T1D (19). Increased frequencies of FOXP3init CD4+ T cells pointed towards increased T cell activation and more vigorous proliferation of naïve CD4+ T cells upon stimulation suggested higher sensitivity to antigenic stimulation during islet autoimmunity onset. The reduced Treg induction in recent onset autoimmunity was attributed to increased miR-181a and nuclear factor of activated T cells 5 (NFAT5) expression in CD4+ T cells. NFAT5 is a transcription factor that plays an important role in T cell activation along with other NFAT members (20). The authors proposed that miR-181a mediated suppression of PTEN promoted PI3k signaling which in turn activated NFAT5 leading to impaired Treg induction and onset of islet autoimmunity in high risk individuals (19). Moreover, increased miR-181a levels promoted CD28 expression which further triggered PI3k signaling leading to NFAT5 activation, thereby providing additional explanation for the observed induction of NFAT5 by miR-181a (Figure 1).
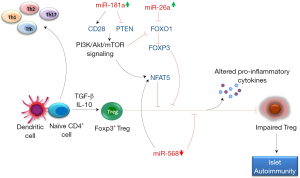
Further investigations in non-obese diabetic (NOD) mice with recent onset of insulin autoantibodies (IAAs) compared to IAA-NOD mice also showed impaired Treg induction, followed by enhanced miR-181a and Nfat5 expression in both naïve CD4+ T cells and ex vivo activated CD4+ T cells (19). Application of miRNA-181a antagomir in IAA+NOD mice enhanced ex vivo PTEN expression while significantly reduced CD28 and NFAT5 expression in CD4+ T cells. Blocking miR-181a significantly increased Treg induction and reduced murine islet autoimmunity as measured by the lowered IAA levels. In contrast, miR-181a mimic significantly lowered Treg induction and enhanced Nfat5 abundance upon stimulation of naïve CD4+ T cells in both human and murine models. The application of NFAT5 inhibitor to IAA+NOD mice increased PTEN and FOXO1 expression which further led to improved Treg induction. Based on these results, the authors next measured the Treg induction potential and abundance of Pten and Foxo1 in CD4+ T cells from NFAT5 knockout (NFAT5ko) mice. An increased Treg induction followed by upregulation of Pten and Foxo1 was observed in activated CD4+ T cells from NFAT5ko mice. Whereas, modulating miR-181a activity had no effects on Treg induction in NFAT5ko T cells. In previous studies, miR-181a has been shown to be involved in T-cell differentiation and tuning the threshold for T-cell receptor (TCR) signaling by downregulating the expression of multiple phosphatases in the TCR signaling pathway (21).
Signals from TCR, PI3K-Akt-mTOR network, IL-2 and TGF-β regulate Treg cell associated FOXP3 expression (22). FOXP3 plays a significant role in conversion of naïve CD4+ T cells to Treg cells. FOXP3 gene harbors multiple NFAT binding sites and several lines of evidence have shown that expression of FOXP3 in TGF-β-induced Treg is highly dependent on NFAT expression (20,22). Further, PI3K-Akt-mTOR signaling inactivates FOXO1/3 which in turn inhibits TGF-β-induced FOXP3 expression leading to Treg deficiency and accelerated autoimmunity (23). In addition to miR-181a, changes in expression levels of miR-26a and miR-568 have been shown to affect CD4+ T cell activation and Treg function by interfering with PI3k signaling, NFAT5 and FOXP3 expression in recent-onset autoimmunity (Figure 1). Increased expression of miR-26a and decreased expression of its target EZH2 was observed in in vivo activated Treg cells contributing to impaired Treg function in pre-T1D (18). Inhibition of EZH2 during activation of Treg cells decreased the expression of immune repressive receptor TIGIT and FOXP3 and also decreased Treg proliferation (18). Li et al. demonstrated that miR-568 inhibited Treg activation, differentiation and their suppressive function by targeting NFAT5 (24). Expression of miRNA-568 decreased while NFAT5 increased in CD4+ T cells during T cell activation. Further, transfection of miR-568 mimics inhibited NFAT5 expression and proliferation of Treg cells (24).
Several miRNAs including miR-17~92 cluster, miR-146a and miR-155 have been identified as important regulators of another subset of CD4+ T cells known as T follicular helper (TFH) cells (25,26). TFH cells play crucial roles in B-cell activation and autoantibody production. In a previous study by Serr et al. increased frequencies of TFH precursors indicative of increased immune activation were observed in nondiabetic children with recent onset islet autoimmunity (<5 years autoantibody positive) compared to persistent (>5 to <10 years autoantibody positive), long-term (>10 years without overt T1D) and no autoimmunity (26). miR-92a was significantly up-regulated in children with recent onset autoimmunity and critically involved in induction of TFH precursors. It is important to note that miR-92a-mediated TFH precursor induction is also regulated by PI3K-Akt-mTOR signaling involving downregulation of PTEN and FOXO1 levels. It is plausible that manipulating PTEN-PI3K signaling or miR-92a levels by antagomir application may reduce THF induction and thereby reduce islet autoimmunity. However, the direct role of TFH cells in promoting the onset of human islet autoimmunity still remains unclear.
The ultimate challenge in preventing T1D has been to get better insights in the early stages of disease progression and identify the initial triggers of islet autoimmunity. The study by Serr et al. (19) and other studies, e.g., those discussed above, have proved that miRNAs play a huge role in impairment of Treg function during recent onset autoimmunity. As determined by Serr et al., targeted inhibition of miR-181a and/or NFAT5 may open up novel approaches for reducing islet autoimmunity (19). The authors also reported up-regulation of several other miRNAs such as miR-92a, miR-146b, miR-101, miR-26a and miR-150 in activated T cells from children with islet autoantibodies compared to no autoantibodies. The effects of these highly upregulated miRNAs on Treg induction remains to be investigated. Additional mechanistic studies involving fine tuning of miRNA expression specifically inhibition of miR-181a, miR-26a and up-regulation of miR-568 in murine and humanized models are highly warranted. Overall, these studies might provide potential cues towards development of translational strategies for improving or restoring immune tolerance of regulatory T cells towards β cells thereby limiting islet autoimmunity.
Acknowledgments
Funding: Related work in the Pociot laboratory receives funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 115797 (INNODIA), which receives support from the European Union’s Horizon 2020 research and innovation programme and “EFPIA”, “JDRF” and “The Leona M. and Harry B. Helmsley Charitable Trust”.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Guoping Li (Cardiovascular Division of the Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ncri.2018.03.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet 2016;387:2331-9. [Crossref] [PubMed]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281-97. [Crossref] [PubMed]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005;120:15-20. [Crossref] [PubMed]
- O’Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol 2012;30:295-312. [Crossref] [PubMed]
- Garo LP, Murugaiyan G. Contribution of MicroRNAs to autoimmune diseases. Cell Mol Life Sci 2016;73:2041-51. [Crossref] [PubMed]
- Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol 2013;9:513-21. [Crossref] [PubMed]
- Zheng Y, Wang Z, Zhou Z. miRNAs: novel regulators of autoimmunity-mediated pancreatic β-cell destruction in type 1 diabetes. Cell Mol Immunol 2017;14:488-96. [Crossref] [PubMed]
- Sebastiani G, Spagnuolo I, Patti A, et al. MicroRNA expression fingerprint in serum of type 1 diabetic patients. Diabetologia 2012;55:S48.
- Nielsen LB, Wang C, Sørensen K, et al. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp Diabetes Res 2012;2012:896362
- Salas-Pérez F, Codner E, Valencia E, et al. MicroRNAs miR-21a and miR-93 are down regulated in peripheral blood mononuclear cells (PBMCs) from patients with type 1 diabetes. Immunobiology 2013;218:733-7. [Crossref] [PubMed]
- Assmann TS, Recamonde-Mendoza M, De Souza BM, et al. MicroRNA expression profiles and type 1 diabetes mellitus: systematic review and bioinformatic analysis. Endocr Connect 2017;6:773-90. [Crossref] [PubMed]
- Yang M, Ye L, Wang B, et al. Decreased miR-146 expression in peripheral blood mononuclear cells is correlated with ongoing islet autoimmunity in type 1 diabetes patients 1miR-146. J Diabetes 2015;7:158-65. [Crossref] [PubMed]
- Sebastiani G, Grieco FA, Spagnuolo I, et al. Increased expression of microRNA miR-326 in type 1 diabetic patients with ongoing islet autoimmunity. Diabetes Metab Res Rev 2011;27:862-6. [Crossref] [PubMed]
- Snowhite IV, Allende G, Sosenko J, et al. Association of serum microRNAs with islet autoimmunity, disease progression and metabolic impairment in relatives at risk of type 1 diabetes. Diabetologia 2017;60:1409-22. [Crossref] [PubMed]
- Åkerman L, Casas R, Ludvigsson J, et al. Serum miRNA levels are related to glucose homeostasis and islet autoantibodies in children with high risk for type 1 diabetes. PLoS One 2018;13:e0191067 [Crossref] [PubMed]
- Kroesen BJ, Teteloshvili N, Smigielska-Czepiel K, et al. Immuno-miRs: critical regulators of T-cell development, function and ageing. Immunology 2015;144:1-10. [Crossref] [PubMed]
- Sharma VK, Kaveri SV, Bayry J. Impaired regulatory T cell function in autoimmune diseases: are microRNAs the culprits? Cell Mol Immunol 2016;13:135-7. [Crossref] [PubMed]
- Zhang Y, Feng ZP, Naselli G, et al. MicroRNAs in CD4(+) T cell subsets are markers of disease risk and T cell dysfunction in individuals at risk for type 1 diabetes. J Autoimmun 2016;68:52-61. [Crossref] [PubMed]
- Serr I, Scherm MG, Zahm AM, et al. A miRNA181a/NFAT5 axis links impaired T cell tolerance induction with autoimmune type 1 diabetes. Sci Transl Med 2018;10. pii: eaag1782.
- Vaeth M, Schliesser U, Müller G, et al. Dependence on nuclear factor of activated T-cells (NFAT) levels discriminates conventional T cells from Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 2012;109:16258-63. [Crossref] [PubMed]
- Li QJ, Chau J, Ebert PJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell 2007;129:147-61. [Crossref] [PubMed]
- Merkenschlager M, von Boehmer H. PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. J Exp Med 2010;207:1347-50. [Crossref] [PubMed]
- Wu Y, Borde M, Heissmeyer V, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell 2006;126:375-87. [Crossref] [PubMed]
- Li W, Kong LB, Li JT, et al. MiR-568 inhibits the activation and function of CD4+ T cells and Treg cells by targeting NFAT5. Int Immunol 2014;26:269-81. [Crossref] [PubMed]
- Maul J, Baumjohann D. Emerging Roles for MicroRNAs in T Follicular Helper Cell Differentiation. Trends Immunol 2016;37:297-309. [Crossref] [PubMed]
- Serr I, Fürst RW, Ott VB, et al. miRNA92a targets KLF2 and the phosphatase PTEN signaling to promote human T follicular helper precursors in T1D islet autoimmunity. Proc Natl Acad Sci U S A 2016;113:E6659-E68. [Crossref] [PubMed]
Cite this article as: Kaur S, Pociot F. miRNAs regulate development and function of regulatory T-cells in recent onset islet autoimmunity in pre-Type 1 diabetes. Non-coding RNA Investig 2018;2:16.


