
Inhibition of miR-29 protects against cardiac hypertrophy and fibrosis: new insight for the role of miR-29 in the heart
In response to a sustained pathological stress, such as chronic pressure overload or myocardial infarction, the heart undergoes maladaptive cardiac remodelling to compensate for the increase in workload, ultimately leading to heart failure if left untreated (1). Heart failure is a significant global health problem which is becoming worse as the population ages (2). Despite advances in pharmacological agents, management of patients with heart failure and surgical improvements, mortality rates remain high. One of the key features of maladaptive cardiac remodelling is the accumulation of collagen in the extracellular matrix (i.e., fibrosis), and most cardiac diseases are associated with fibrosis in the heart. Fibrosis is a process whereby heart tissue is progressively replaced with scar tissue leading to stiffness of the heart. The deposition of excessive extracellular matrix proteins by fibroblasts has a significant effect on reducing heart contractility leading to declining heart function and adverse cardiovascular events (3). No effective therapies specifically targeting cardiac fibrosis are available, highlighting that the development of novel and efficacious therapies that can resolve or prevent fibrosis are of paramount importance for the treatment of patients that suffer from life-threatening heart disease. Since the emergence of microRNAs (miRNAs) as important regulators in cardiac pathology [see reviews (4-6)], including fibrosis (7-10), their potential as therapeutic targets for heart disease has been well explored in preclinical studies in cardiac disease settings (11-15). miRNAs are evolutionarily-conserved, short strands of RNA and do not code for protein. miRNAs are able to regulate the expression of hundreds of genes by interacting with specific sites in the 3’untranslated regions of messenger transcripts to prevent protein translation and gene expression (4,5). Drugs targeting miRNAs have been developed and tested in clinical trials to understand safety and tolerability in patients with hepatitis C virus or cancer (5), demonstrating the therapeutic potential of miRNA-targeted drugs.
In a recent issue of Nature Communications, Sassi and colleagues have focused on the role of miR-29 in regulating pathological cardiac remodelling (16). Strong anti-fibrotic effects of miR-29 family members have previously been demonstrated in heart, kidney, and other organs, and thus, has been the focus of several studies [see review (17)]. The miR-29 family has four closely related precursors: MiR-29a, MiR-29b-1, MiR-29b-2, and MiR-29c which are expressed as two bicistronic clusters (miR-29a/miR-29b-1 and miR-29b-2/miR-29c). As miR-29b-1 and miR-29b-2 have identical mature sequences, they are called miR-29b (Figure 1). All family members share a conserved seed region and are highly conserved in human, mouse, and rat (18). In both rodents and humans, the expression of miR-29 has been reported to be downregulated in fibrosis-related disorders, and shown to negatively regulate a plethora of mRNAs encoding collagens and extracellular matrix proteins in different cell types, suggesting that enhancing miR-29 would be a promising anti-fibrotic therapy (17). However, despite data suggesting that increasing miR-29 could be a promising therapeutic strategy in the diseased heart by inhibiting cardiac fibrosis, Jentzsch et al., had identified miR-29 as an inducer of cardiomyocyte hypertrophy from a phenotypic screen (19). The role of miR-29 in regulating cardiomyocyte hypertrophy and cardiac hypertrophy had not previously been examined in detail. In order for miR-29 to enter the clinic as an anti-fibrotic therapy, it is important to thoroughly analyse its function experimentally. This is important as miR-29 family members are regulated by distinct mechanisms and have different subcellular distribution, suggesting they may not function in an identical manner. For this purpose, Sassi et al., undertook a very comprehensive study incorporating genetic mouse models, miRNA inhibitors, gene therapy, cell culture, and secretome analysis to assess the role of miR-29 in regulating cardiomyocyte hypertrophy and fibrosis (Figure 1) (16).
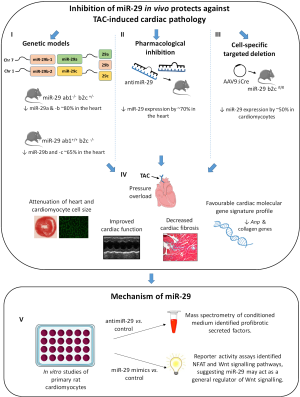
Sassi and colleagues (16) report that miRNA-29 promotes pathological hypertrophy of cardiac myocytes, cardiac dysfunction and increased cardiac fibrosis, suggesting that the effects of miR-29 in cardiac myocytes are different to that in cardiac fibroblasts. After demonstrating in vitro that overexpression of miR-29a, -b and -c promoted hypertrophy of neonatal rat cardiomyocytes, to validate the importance of miR-29 in cardiac remodelling, the authors generated a genetic mouse model with triple-allelic partial deletion of miR-29 clusters (mice with complete genetic loss of miR-29 clusters die shortly after birth). They compared two genetic mouse models with partial deficiency of miR-29 variants in their study: (I) miR-29 ab1-/-b2c+/- and miR-29 ab1+/+ b2c-/-. A reduction of between 65–80% of miR-29 expression levels in the hearts of these mice was confirmed by qPCR (Figure 1). Under basal conditions, both genetic mouse models have no overt cardiac abnormalities. That is, there was no difference in cardiac function, heart size, cardiomyocyte size or fibrosis. This is not surprising as others have reported similar observations in miRNA knockout mice (9) or miRNA transgenic mice (20) under basal conditions. However, when the miR-29 knockout mouse lines were subjected to a pathological cardiac stress for three weeks [pressure overload induced by transverse aortic constriction (TAC)], both lines were protected from TAC-induced cardiac pathology. TAC-induced cardiac hypertrophy was attenuated in miR-29 ab1-/-b2c+/- and miR-29 ab1+/+ b2c-/- mice compared to wildtype TAC mice (as assessed by both heart weight and myocyte cell size). Further, following TAC and compared to wildtype mice, both miR-29 mouse lines had enhanced cardiac function and decreased cardiac fibrosis. In addition, these mice had an improved cardiac molecular gene signature with decreased expression of genes associated with pathological hypertrophy (atrial natriuretic peptide) and fibrosis (collagens 1, 2 and 3) (Figure 1). To rule out any compensatory mechanisms in the miR-29 ab1-/-b2c+/- and miR-29 ab1+/+ b2c-/- mice, the authors next turned to chemically modified antisense oligonucleotide technology to inhibit the miR-29 family acutely in the adult heart. Using single-stranded miRNA inhibitors with locked nucleic acid (LNA) chemistry (i.e., antimiR-29), Sassi and colleagues (16) inhibited all three miR-29 family members in the heart by ~70%. Similar to the mouse deficient genetic studies, pharmacological inhibition of the miR-29 family prevented cardiac remodelling and cardiac dysfunction following TAC-induced pressure overload. Specifically, treatment with antimiR-29 attenuated TAC-induced cardiac hypertrophy and cardiomyocyte cell size, and cardiac function was improved when compared to control TAC mice. Improved cardiac function in antimiR-29 treated TAC mice was associated with decreased fibrosis and decreased expression of pathological and fibrotic genes (Figure 1). It is noteworthy that these findings by Sassi and colleagues (16) are in direct contrast to earlier studies (7,21). An earlier study reported that despite no significant change in miR-29b with TAC, treatment of mice 3 days prior to TAC with antagomiRs to miR-29b induced excess perivascular fibrosis after 2 weeks of aortic constriction, this data was not quantitated (21). Similarly, under basal conditions, inhibiting miR-29b with an antagomiR increased cardiac collagen gene expression analysed by real time PCR (not histologically), although this would need to be confirmed in a larger cohort, as only 2 animals per group were analysed (7). The effect of miR-29b inhibition or overexpression following a cardiac insult was not investigated in the study (7). Two major differences between these studies may explain the apparent disparity: (I) different oligonucleotide chemistries; and (II) specific targeting of miR-29b vs. miR-29 family. Sassi and colleagues (16) used LNA-modified inhibitors, whereas the study of Abonnenc et al. (21). and Van Rooij et al. (7) utilised cholesterol conjugated inhibitors. Opposing outcomes targeting the same miRNA with different antisense oligonucleotide chemistries has been reported previously (9,10). Secondly, specific targeting of miR-29b (7,21) as opposed to the miR-29 family (16) may have a different function to that of miR-29-a and -c. Studies performed in cardiac mouse disease models have reported that inhibition of a miRNA family has more therapeutic beneficial than an individual miRNA (11,22), and disease severity and sex influences therapeutic outcome (22,23). Finally, given that miRNAs have been shown to regulate other miRNAs (24), an understanding of miRNA regulatory networks is integral to the successful design of miRNA based targeted therapy.
In light of these unresolved discrepancies surrounding miR-29, Sassi and colleagues (16) next investigated the expression of miR-29 family members in healthy and diseased hearts, and the relative abundance in cardiac myocytes and fibroblasts. The expression of miR-29a, -b and -c increased with age in mice. However, what was most interesting was the dynamic change of miR-29 family members in the hearts of mice in response to TAC. miR-29a, -b and -c were upregulated 1 and 2 days after TAC, but expression decreased at 14 days post TAC and further declined at 21 days post TAC. When myocytes and fibroblasts were freshly isolated from adult mice, miR-29a, -b and -c were expressed 6-fold higher in cardiomyocytes compared to cardiac fibroblasts. Although this finding is different to a previous report where miR-29 family members were highly expressed in rat neonatal cardiac fibroblasts compared to myocytes (7), the authors suggest the different findings could be due to culturing conditions and cultivation (16). Finally, the expression of miR-29a and -b was downregulated in patients with aortic valve stenosis compared to healthy controls, whereas the expression of miR-29c was unchanged.
To further explore the role of miR-29 specifically in cardiomyocytes, Sassi and colleagues (16) used an adeno associated virus (AAV) approach to selectively target cardiomyocytes in the adult mouse heart (Figure 1). To target miR-29 in cardiomyocytes, they used the AAV9 serotype (this serotype has shown cardiac tropism) to deliver improved Cre recombinase (AAV9:iCre) to mice that carry floxed alleles of the miR-29 b2c cluster. This technique resulted in a 50% decrease of the expression of miR-29b and -c in the hearts of mice. Treatment of floxed miR-29b2c mice with AAV9:iCre prevented cardiac hypertrophy, cardiac dysfunction and fibrosis following 3 weeks of TAC (Figure 1). This phenotype mirrored that of genetic deletion or pharmacological inhibition of miR-29. In contrast, cardiomyocyte specific miR-29a overexpression (using AAV9-miR-29a) led to enhanced cardiomyocyte hypertrophy and a moderate increase in fibrosis in response to pressure overload. These comprehensive studies suggest a role of miR-29 in cardiomyocytes.
Further supporting the profibrotic role of miR-29, mass spectrometry analysis of the secretome of primary cardiac myocytes after transfection with antimiR-29 or control identified several proteins with profibrotic actions that were downregulated in antimiR-29 treated cardiomyocytes (Figure 1). Mechanistically, the downregulated factors had binding sites enriched for T-cell factor/lymphoid enhancer factor (TCF/LEF) and nuclear factor of activated T-cells (NFAT), which are endpoint genes in the Wnt/frizzled signalling pathway. This is of particular interest as Wnt signalling controls not only heart development, but is also modulated during adult heart remodelling in response to a pathological stress (25). Sassi and colleagues (16) confirm that overexpression of miR-29 in cultured cardiomyocytes activated both Wnt and NFAT signalling pathways, and in vitro studies confirmed miR-29 acts as a general regulator of Wnt signalling (Figure 1). Specifically, four factors in the Wnt signalling pathway (Glis2, Gsk3b, Hbp1 and Ctnnbip1) were identified and validated as direct target genes of miR-29. Finally, they show that inhibition of Wnt and NFAT signalling in vitro abolished miR-29-induced cardiomyocyte hypertrophy, demonstrating that miR-29 regulates canonical and non-canonical Wnt signalling in cardiomyocytes.
Taken together, the combined studies of (I) the genetic deficient mouse model of miR-29; (II) pharmacological inhibition of the miR-29 family; and (III) the use of gene therapy by AAV to delete miR-29 specifically in cardiomyocytes all elegantly demonstrate that inhibition of miR-29 protects against pressure-overload induced cardiac remodelling, and that this protection, in part, may be mediated by Wnt signalling in cardiomyocytes.
How do these confounding studies influence the development of miR-29 as a therapy for the treatment of heart failure? Firstly, it would be important to determine timing and duration of miR-29 inhibition therapy, given that miR-29 expression levels are dynamically regulated in a setting of TAC. Secondly, as miRNA inhibitors (LNA or cholesterol conjugated) are not tissue specific and taken up by other tissues upon systemic administration, and have a reported profibrotic effect in other organs (e.g., lung, kidney, liver) it would be imperative to develop a miR-29 therapy that directly targets the heart, and specific cells within the heart. This may be achieved with AAV vectors with specific promoters and reporter genes, or incorporation of antimiRs with liposomes or nanoparticles. Liposomes and nanoparticles can be modified to include cell surface receptors or antibodies to allow for cell- or tissue-specific delivery of miRNAs in vivo. However, these technologies are still being developed and tested (5). If successful, the application of these different approaches targeting miRNAs could yield a new generation of drugs for the treatment of heart failure.
Acknowledgments
Funding: J.R.M is a National Health and Medical Research Council Senior Research Fellow (APP1078985), and B.C.B is supported by an Alice Baker and Eleanor Shaw Fellowship (The Baker Foundation, Melbourne, Australia).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Dr. Rongrong Gao (Department of Cardiology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ncri.2018.03.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bernardo BC, Weeks KL, Pretorius L, et al. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther 2010;128:191-227. [Crossref] [PubMed]
- Braunwald E. The war against heart failure: the Lancet lecture. Lancet 2015;385:812-24. [Crossref] [PubMed]
- Travers JG, Kamal FA, Robbins J, et al. Cardiac Fibrosis. Circ Res 2016;118:1021-40. [Crossref] [PubMed]
- Bernardo BC, Charchar FJ, Lin RCY, et al. A MicroRNA Guide for Clinicians and Basic Scientists: Background and Experimental Techniques. Heart Lung Circ 2012;21:131-42. [Crossref] [PubMed]
- Bernardo BC, Ooi JYY, Lin RCY, et al. miRNA therapeutics: a new class of drugs with potential therapeutic applications in the heart. Future Med Chem 2015;7:1771-92. [Crossref] [PubMed]
- Hata A. Functions of MicroRNAs in Cardiovascular Biology and Disease. Annu Rev Physiol 2013;75:69-93. [Crossref] [PubMed]
- van Rooij E, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A 2008;105:13027-32. [Crossref] [PubMed]
- Matkovich SJ, Wang W, Tu Y, et al. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res 2010;106:166-75. [Crossref] [PubMed]
- Patrick DM, Montgomery RL, Qi X, et al. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest 2010;120:3912-6. [Crossref] [PubMed]
- Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980-4. [Crossref] [PubMed]
- Bernardo BC, Gao XM, Winbanks CE, et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci U S A 2012;109:17615-20. [Crossref] [PubMed]
- Bernardo BC, Nguyen SS, Gao XM, et al. Inhibition of miR-154 Protects Against Cardiac Dysfunction and Fibrosis in a Mouse Model of Pressure Overload. Sci Rep 2016;6:22442. [Crossref] [PubMed]
- Bernardo BC, Nguyen SS, Winbanks CE, et al. Therapeutic silencing of miR-652 restores heart function and attenuates adverse remodeling in a setting of established pathological hypertrophy. FASEB J 2014;28:5097-110. [Crossref] [PubMed]
- Hullinger TG, Montgomery RL, Seto AG, et al. Inhibition of miR-15 Protects Against Cardiac Ischemic Injury / Novelty and Significance. Circ Res 2012;110:71-81. [Crossref] [PubMed]
- Tao L, Bei Y, Chen P, et al. Crucial Role of miR-433 in Regulating Cardiac Fibrosis. Theranostics 2016;6:2068-83. [Crossref] [PubMed]
- Sassi Y, Avramopoulos P, Ramanujam D, et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat Commun 2017;8:1614. [Crossref] [PubMed]
- He Y, Huang C, Lin X, et al. MicroRNA-29 family, a crucial therapeutic target for fibrosis diseases. Biochimie 2013;95:1355-9. [Crossref] [PubMed]
- Kriegel AJ, Liu Y, Fang Y, et al. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics 2012;44:237-44. [Crossref] [PubMed]
- Jentzsch C, Leierseder S, Loyer X, et al. A phenotypic screen to identify hypertrophy-modulating microRNAs in primary cardiomyocytes. J Mol Cell Cardiol 2012;52:13-20. [Crossref] [PubMed]
- Liu X, Xiao J, Zhu H, et al. miR-222 Is Necessary for Exercise-Induced Cardiac Growth and Protects against Pathological Cardiac Remodeling. Cell Metab 2015;21:584-95. [Crossref] [PubMed]
- Abonnenc M, Nabeebaccus AA, Mayr U, et al. Extracellular matrix secretion by cardiac fibroblasts: role of microRNA-29b and microRNA-30c. Circ Res 2013;113:1138-47. [Crossref] [PubMed]
- Bernardo BC, Gao XM, Tham YK, et al. Silencing of miR-34a attenuates cardiac dysfunction in a setting of moderate, but not severe, hypertrophic cardiomyopathy. PLoS One 2014;9:e90337 [Crossref] [PubMed]
- Bernardo BC, Ooi JYY, Matsumoto A, et al. Sex differences in response to miRNA-34a therapy in mouse models of cardiac disease: Identification of sex-, disease- and treatment-regulated miRNAs. J Physiol 2016;594:5959-74. [Crossref] [PubMed]
- Ooi J, Bernardo B, Lin R, et al. Identification of novel cardioprotective miRNAs and mRNAs regulated by miR-34. Heart Lung Circ 2015;24:S179. [Crossref]
- Bergmann MW. WNT signaling in adult cardiac hypertrophy and remodeling: lessons learned from cardiac development. Circ Res 2010;107:1198-208. [Crossref] [PubMed]
Cite this article as: McMullen JR, Bernardo BC. Inhibition of miR-29 protects against cardiac hypertrophy and fibrosis: new insight for the role of miR-29 in the heart. Non-coding RNA Investig 2018;2:14.


