
A deeper exploration of the relationship between the miR-27a and insulin resistance
Type 2 diabetes mellitus (T2DM) is an increasing healthcare concern, data from the International Diabetes Federations Atlas (2015) reports over 415 million people have been diagnosed with diabetes (1). By 2040 this figure is expected to reach 642 million people with nearly 90% of diabetes cases being T2DM (1). While there are a variety of underlying factors contributing to the onset of T2DM, obesity is one of the main risk factors leading to the development of T2DM (2). Obesity induced insulin resistance causing dysfunction of insulin signaling and resistance to insulin in skeletal muscle are key contributors to the onset of T2DM (3,4). The complex pathogenesis associated with adipose tissue in obese individuals generates systemic effects through factors including local inflammation of white adipose tissue, cytokine responses, and immune cell infiltrates (2). Additionally, factors such as adipose-derived adipokines and adipocyte-induced macrophage polarization have been shown to influence skeletal muscle insulin resistance through crosstalk signaling mechanisms (5,6). Although crosstalk signaling mediated through secreted non-coding microRNAs (miRNAs) from adipose tissue have received less attention.
Non-coding RNAs are of increasing interest due to their diverse biological effects on gene expression. Coding RNA translated into protein represents a small minority of the total RNA, roughly 75% of the genome is transcribed into RNA but only 2% of this transcribed RNA goes on to be translated into protein (7). While the fate of coding RNA is well established, the fate of non-coding RNA continues to expand and evolve. One type of non-coding RNA is miRNA, these single stranded RNA sequences are 21−25 nucleotides in length and can negatively regulate gene expression at the translational level by hybridizing with messenger RNA (mRNA) sequences. These miRNAs can be secreted from cells in exosomes and taken up by distant cells where they can influence recipient cell protein translation. Adipose tissue is one of major sources of exosomal miRNAs, and therefore of increased interest in the context of obesity. One of the major miRNAs expressed in adipose tissue is miR-27a, initially proposed as a negative regulator of adipogenic and lipogenic pathways (8,9) the role of miR-27a continues to expand. Recently, in adipocytes miR-27a was shown to act as a negative regulator of peroxisome proliferator-activated receptor γ (PPARγ) (8,10), a transcription factor known to modulate lipid metabolism and insulin sensitivity. With miR-27a being a major miRNA expressed in adipose tissue, adipose tissue being a source of exosomal miRNAs, and the recent data showing miR-27a can negatively regulate PPARγ, a major modulator of systemic insulin sensitivity, sets the stage for miR-27a potentially having a greater role than initially thought.
The recent study from Yu et al. identifies a mechanism of crosstalk signaling between adipose tissue and skeletal muscle mediated by miR-27a and its influence on promoting insulin tolerance. While negative regulation of PPARγ by miR-27a has been reported (8,10), the ability of adipocyte derived miR-27a to negatively regulate PPARγ and contribute to insulin resistance in skeletal muscle remained unknown. The first critical link to establishing this connection is the ability for miR-27a to be transported from adipose tissue to skeletal muscle, and therefore the detection of miR-27a in serum. Microarray analysis have previously identified increased serum miR-27a levels show a positive correlation with the level of fasting glucose in obese and obese-related T2DM patients (11). In addition, a positive correlation between serum miR-27a levels and BMI has been reported in adults (12). Building on these findings, the authors examined miR-27a levels in obese and non-obese children, showing a positive correlation between serum miR-27a levels and BMI. Extending their studies to mouse models, the authors have previously reported a correlation between miR-27a and insulin resistance in high-fat diet induced obese mice (HF mice) (13). In this study, the authors utilized their established high-fat diet induced obese mice with an additional genetically engineered mouse model for T2DM, the leptin receptor-deficient db/db mice. In both mouse models, the authors show increased serum miR-27a levels compared to control mice. In effort to correlate the increased serum levels with insulin resistance, the authors show when miR-27a was systemically knocked down in HF obese mice the result was decreased insulin resistance. Combined, the authors indicate the correlation in serum miR-27a levels suggest its potential role as a signaling mediator.
With the established correlation between obesity and serum miR-27a levels (Figure 1), the authors propose the increase in serum miR-27a levels could be derived from exosomes secreted from adipose tissue. This position was motivated by the following observations; adipose tissue is the dominant source of exosomal miRNAs, recent reports indicate adipocyte-derived exosomes may contribute toward developing insulin resistance in obese states (14,15), and that miR-27a is highly expressed in adipose tissue (8,9). Therefore, serum exosomes were examined for FABP4, an adipocyte-derived exosome marker. The authors report the increased miR-27a levels from HF mice were associated with increased FABP4 exosomes and FABP4 exosomes colocalized with miR-27a in the serum, a finding that supports adipose tissue as the source of miR-27a. Interestingly, when the authors examined visceral adipose tissue of HF mice, they observed an initial increase in miR-27a levels at 2 weeks, but after this time point levels of miR-27a were lower compared to low-fat diet control mice. This observation would seem to contradict the idea that adipose tissue is the source of miR-27a. Although, the authors demonstrate adipose tissue is indeed the source of miR-27a and identify the reason for the decrease in visceral adipose tissue miR-27a levels after the 2-week time point. In HF mice, the timing of the increased serum levels of miR-27a correlates with the decreased miR-27a levels in visceral adipose tissue, indicating the decreased levels in visceral adipose tissue is the result of increased secretion of miR-27a into circulation. The increased secretion of miR-27a into circulation was proposed to have a reciprocal effect lowering the levels of miR-27a in visceral adipose tissue. To confirm this observation, differentiated 3T3-L1 cells were utilized in the presence and absence of palmitate to mimic lipid drop accumulation in adipose tissue of HF mice. Then expression and secretion of miR-27a were determined, and the data again revealed a similar finding to what was observed in vivo with visceral adipose tissue. When 3T3-L1 cells were treated with palmitate there was a decrease in miR-27a levels in the cells, although there was a nearly a 3-fold increase in miR-27a in the supernatant. This observation provides evidence that increased secretion of miR-27a results in lower intracellular miR-27a levels. Since adipose tissue associated macrophage can be a source of exosomal miRNA, the authors extended these studies to examine RAW264.7 macrophage as a source of secreted miR-27a. Following treatment with palmitate to again mimic the high fat obese state, the miR-27a levels were increased within the macrophage but there was no increase in secreted miR-27a, as determined by levels in the supernatant. With evidence supporting the source of increased miR-27a levels being adipocyte derived exosomal, moving forward the authors sought to establish the effect on skeletal muscle.
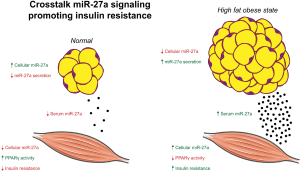
Skeletal muscle is the cornerstone of glucose homeostasis, as the major tissue for the disposal of consumed glucose in normal individuals’, dysfunction in this role results in systemic insulin resistance (16). The authors report insulin resistance in HF mice supported by the decreased expression of insulin signaling proteins and decreased insulin dependent glucose uptake activity that were mitigated by the systemic knock down of miR-27a. Therefore, they further investigated the effect of miR-27a on skeletal muscle and myoblasts. Previous studies have shown the combined silencing of miR-106b, miR-30d, and miR-27a in L6 skeletal muscle cells led to enhanced glucose uptake and consumption (17). This is complemented by the authors demonstrating that overexpression of miR-27a in the myoblast cell line C2C12 inhibited glucose uptake and consumption. Although, the potential of adipose tissue derived exosomal miR-27a to function as a modulator of skeletal muscle has remained unknown. The obvious critical link to adipose tissue derived exosomal miR-27a influencing skeletal muscle is the ability for the exosomal miR-27a to be taken up in skeletal muscle cells. The authors provide evidence of adipose derived exosome uptake by detecting increased levels of FABP4 in skeletal muscle of HF mice. While adipose tissue can secrete an array of exosomal miRNAs the detection of increased FABP4 in skeletal muscle was associated with increased levels of miR-27a within skeletal muscle cells. Further in vitro evidence was provided from C2C12 cells incubated with conditioned media from 3T3-L1 cells treated with palmitate to generate exosomal miR-27a. Following treatment with the conditioned media containing exosomal miR-27a, C2C12 cells showed elevated intracellular miR-27a levels. The combined in vivo and in vitro work supports the hypothesis that increased miR-27a in skeletal muscle in the obese state has adipose tissue origins and is transported to skeletal muscle as adipose derived exosomal miR-27a.
Yu and colleagues have outlined the physiological basis for miR-27a to induce insulin resistance in skeletal muscle. This is supported by the association of serum levels with obesity and the resulting increase in secreted exosomal miR-27a from adipose tissue that is taken up by skeletal muscle leading to elevated levels of miR-27a. The authors then focus on identifying the cellular mechanism that miR-27a induces insulin resistance in skeletal muscle. The focus of these cellular mechanistic studies was directed towards PPARγ since miR-27a has been shown as a negative regulator of PPARγ (8,10). It is known that PPARγ has a key role in skeletal muscle insulin resistance by influencing glucose uptake (18), although the effect of miR-27a on PPARγ in skeletal muscle has not been examined. Previous reports have illustrated that induction of PPARγ expression in adipocytes improves insulin resistance in obese mice (19), and skeletal muscle specific knockouts of PPARγ results in progressive insulin resistance (20). In addition, thiazolidinediones (TZDs) such as rosiglitazone function as PPARγ-dependent trans-activators and are used clinically as insulin sensitizers (21). Using C2C12 cells overexpressing miR-27a the authors show miR-27a reduces glucose uptake and consumption, and treatment with rosiglitazone partially reverses this effect. In addition, C2C12 cells overexpressing miR-27a treated with rosiglitazone increased mRNA expression of not only PPARγ but also insulin receptor substrate 1 (IRS-1) and GLUT4 to further improve insulin resistance.
Collectively, through a combination of physiological and cellular investigations the study by Yu and colleagues expands the current understanding of crosstalk signaling between adipose tissue and skeletal muscle. In the context of obesity-induced T2DM, the authors have highlighted the influence of miR-27a on insulin resistance and contributed a step forward towards our understanding of the diverse and expanding biological functions of miRNAs. Future studies using short (22-24) and long molecules (25) to regulate the expression of miR-27a can further expand on potential therapeutic opportunities.
Acknowledgments
Funding: DPA thanks the NIH (AI 120303) for financial support.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Dr. Rongrong Gao, Section Editor (Department of Cardiology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ncri.2018.09.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Riddy DM, Delerive P, Summers RJ, et al. G Protein-Coupled Receptors Targeting Insulin Resistance, Obesity, and Type 2 Diabetes Mellitus. Pharmacol Rev 2018;70:39-67. [Crossref] [PubMed]
- Maassen JA, Romijn JA, Heine RJ. Fatty acid-induced mitochondrial uncoupling in adipocytes as a key protective factor against insulin resistance and beta cell dysfunction: a new concept in the pathogenesis of obesity-associated type 2 diabetes mellitus. Diabetologia 2007;50:2036-41. [Crossref] [PubMed]
- Gallagher EJ, Leroith D, Karnieli E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. Mt Sinai J Med 2010;77:511-23. [Crossref] [PubMed]
- Broussard JL, Castro AB, Iyer M, et al. Insulin access to skeletal muscle is impaired during the early stages of diet-induced obesity. Obesity 2016;24:1922-8. [Crossref] [PubMed]
- Meijer RI, Bakker W, Alta C, et al. Perivascular adipose tissue control of insulin-induced vasoreactivity in muscle is impaired in db/db mice. Diabetes 2013;62:590-8. [Crossref] [PubMed]
- dos Santos B, Estadella D, Hachul AL, et al. Effects of a diet enriched with polyunsaturated, saturated, or trans fatty acids on cytokine content in the liver, white adipose tissue, and skeletal muscle of adult mice. Mediators Inflamm 2013;2013:594958 [Crossref] [PubMed]
- Sana J, Faltejskova SJ, Svoboda M, et al. Novel classes of non-coding RNAs and cancer. J Transl Med 2012;10:103. [Crossref] [PubMed]
- Kim SY, Kim AY, Lee HW, et al. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochem Biophys Res Commun 2010;392:323-8. [Crossref] [PubMed]
- Kajimoto K, Naraba H, Iwai N. MicroRNA and 3T3-L1 pre-adipocyte differentiation. RNA 2006;12:1626-32. [Crossref] [PubMed]
- Li S, Li J, Fei B, et al. MiR-27a promotes hepatocellular carcinoma cell proliferation through suppression of its target gene peroxisome proliferator-activated receptor gamma. Chin Med J (Engl) 2015;128:941-7. [Crossref] [PubMed]
- Villard A, Marchand L, Thivolet C, et al. Diagnostic Value of Cell-free Circulating MicroRNAs for Obesity and Type 2 Diabetes: A Meta-analysis. J Mol Biomark Diagn 2015;6:251. [Crossref] [PubMed]
- Nunez Lopez YO, Garufi G, Seyhan AA. Altered levels of circulating cytokines and microRNAs in lean and obese individuals with prediabetes and type 2 diabetes. Mol Biosyst 2016;13:106-21. [Crossref] [PubMed]
- Yao F, Yu Y, Feng L, et al. Adipogenic miR-27a in adipose tissue upregulates macrophage activation via inhibiting PPARgamma of insulin resistance induced by high-fat diet-associated obesity. Exp Cell Res 2017;355:105-12. [Crossref] [PubMed]
- Thomou T, Mori MA, Dreyfuss JM, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017;542:450-5. [Crossref] [PubMed]
- Aswad H, Forterre A, Wiklander OP, et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia 2014;57:2155-64. [Crossref] [PubMed]
- Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol 2010;2010:476279 [Crossref] [PubMed]
- Zhou T, Meng X, Che H, et al. Regulation of Insulin Resistance by Multiple MiRNAs via Targeting the GLUT4 Signalling Pathway. Cell Physiol Biochem 2016;38:2063-78. [Crossref] [PubMed]
- Ahmadian M, Suh JM, Hah N, et al. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med 2013;19:557-66. [Crossref] [PubMed]
- Soares FL, de Oliveira Matoso R, Teixeira LG, et al. Gluten-free diet reduces adiposity, inflammation and insulin resistance associated with the induction of PPAR-alpha and PPAR-gamma expression. J Nutr Biochem 2013;24:1105-11. [Crossref] [PubMed]
- Hevener AL, He W, Barak Y, et al. Muscle-specific Pparg deletion causes insulin resistance. Nat Med 2003;9:1491-1497. [Crossref] [PubMed]
- Karak M, Bal N, Bal C, et al. Targeting peroxisome proliferator-activated receptor gamma for generation of antidiabetic drug. Curr Diabetes Rev 2013;9:275-85. [Crossref] [PubMed]
- Ghosh A, Degyatoreva N, Kukielski C, et al. Targeting miRNA by tunable small molecule binders: peptidic aminosugar mediated interference in miR-21 biogenesis reverts epithelial to mesenchymal transition. Medchemcomm 2018;9:1147-54. [Crossref] [PubMed]
- Nahar S, Ranjan N, Ray A, et al. Potent inhibition of miR-27a by neomycin-bisbenzimidazole conjugates. Chem Sci 2015;6:5837-46. [Crossref] [PubMed]
- Watkins D, Jiang L, Nahar S, et al. A pH Sensitive High-Throughput Assay for miRNA Binding of a Peptide-Aminoglycoside (PA) Library. PLoS One 2015;10:e0144251 [Crossref] [PubMed]
- Yan LX, Wu QN, Zhang Y, et al. Knockdown of miR-21 in human breast cancer cell lines inhibits proliferation, in vitro migration and in vivo tumor growth. Breast Cancer Res 2011;13:R2. [Crossref] [PubMed]
Cite this article as: Kellish P, Arya DP. A deeper exploration of the relationship between the miR-27a and insulin resistance. Non-coding RNA Investig 2018;2:58.


