
Lactosylated arginine-dehydrophenylalanine nanoparticles increase the selective delivery of miR-199a-3p to liver tumor cells enhancing antitumoral activity
MicroRNAs (miRNAs) are small, non-coding, single-stranded RNAs of about 22 nucleotides in length. They are post-transcriptional modulators of gene expression that act predominantly through the degradation of their target mRNAs. They can affect cellular proteins levels in diverse signaling pathways through the regulation of cancer related genes involved in cell cycle progression, apoptosis, tumor angiogenesis and metastasis (1). Several human conditions, including liver cancer, are characterized by aberrant expression of miRNA. Among deregulated miRNAs in hepatocellular carcinoma (HCC), miR-199a-3p is one of the most relevant (2). Low levels of miR-199a-3p are present both in tissue and serum samples of HCC patients (3), whereas overexpression of miR-199a-3p is found in normal liver tissue (4).
MiR-199a-3p targets multiple pathways
Several studies have investigated its mechanism of action in tumorigenesis and described its target genes. MiR-199a-3p inhibits cell growth through the down-regulation of hypoxia-inducible factor 1-α subunit (HIFα) (5) and p21 activated kinase 4 (PAK4) (6) or induces cellular apoptosis through Yes-associated protein 1 (YAP1) inhibition (7). In both HCC tissues and HCC cell lines, such as HepG2 and SNU449, miR-199a-3p suppresses tumor growth, migration, invasion and angiogenesis by targeting vascular endothelial growth factor A (VEGFA) and its receptors (VEGFR1 and VEGFR2), hepatocyte growth factor (HGF) and matrix metallopeptidase 2 (MMP2) (8). MiR-199a-3p also targets the Notch1/E-cadherin axis decreasing tumor aggressiveness (9), and reducing in vitro invasion of HCC cell lines (10), and CD151, which is implicated in HCC invasion and metastasis (11). Transfection of HCC cell lines with miR-199a-3p decreased CD151 mRNA and protein levels with resulting inhibition of cell migration and invasion (12). Restoration of miR-199a-3p in HCC cell lines lead to reduce invasiveness, by arresting G1-phase cell cycle, through the control of the rapamycin mTOR and MET proto-oncogene (2). NOD/SCID mice injected with SNU449 cells overexpressing premiR-199a-3p displayed reduction of angiogenesis when compared with cells transfected with control vector (8). All together, these findings suggest that miR-199a-3p has an important role in inhibiting HCC progression. MiR-199a-3p delivery into HCC tissue, by restoring cellular levels, could provide an innovative therapeutic approach for this cancer.
Selective delivery system for HCC cells improves miRNAs uptake
A key issue in designing a targeted delivery in a tumor tissue is its efficiency in delivering the bioactive molecule. So far, physical approaches, like electroporation, viral or liposomal vector, were used to deliver miRNA. However, there are several problems in the clinical development of miRNA delivery, which have so far greatly limited their use as a therapeutic option. Among these, scarce cellular selectivity, poor penetration in tumor tissue, rapid degradation, occurrence of neuro- or immune toxicity (13,14) induced by low in vivo stability, and insufficient gene silencing (Figure 1A).
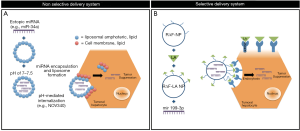
Recently, a new peptide-based nanoparticles (NPs) technology was developed to delivering miRNA in the tissues. This system is attractive because it is biocompatible, biodegradable and easy to synthetize; however, it is susceptible to enzymatic degradation thus preventing safe clinical use (15). In the April issue of Hepatology 2018, Varshney et al. (16) described the synthesis and characterization of a specific NPs carrier containing the short cationic dipeptide arginine α,β‐dehydrophenylalanine (RΔF) in the attempt of making it more resistant to enzymatic degradation. To make the system selective for HCC cells, the authors have exploited the abundant presence on hepatocytes of an asialoglycoprotein receptor (ASGPR), which is instead minimally expressed on extra-hepatic cells (17). Of the various known ligands described for ASGPR, the authors have selected lactobionic acid (LA), which was conjugated with a short cationic peptide containing RΔF. The resulting self-assembling uniform spherical nanostructures (RΔF-LA NPs) efficiently condensed miR-199a-3p, giving rise to a cargo, which was stable in serum and after RNase treatment for up to 6 hours (Figure 1B). By flow cytometry, RΔF-LA/miR NPs has a higher uptake than RΔF/miR NPs but a reduced uptake in lactose pre-treated cells, suggesting a direct involvement of receptor in delivery.
Using in vitro experiments on Huh7 cells, the authors have demonstrated that RΔF-LA/miR NPs has an enhanced cellular uptake, giving rise to more than 500 fold increase in miR-199a-3p tissue levels, RΔF-LA/miR NPs also significantly suppressed mTOR target gene expression both mRNA and protein levels, reduced proliferation and cell migration and induced cellular apoptosis. Similar results were obtained in a xenograft mouse model in which the NPs were introduced by intravenous administration.
Inhibition of cell proliferation, cellular migration, tumor invasion, metastasis and angiogenesis as well as down-regulation of mTOR, VEFGA and MMP2 expression were significantly higher in treated than in the control mice. Most importantly, RΔF-LA/miR NPs treated mice showed an extremely relevant regression of tumor volume in comparison with sham-treated animals. On this line, recently Li et al. (18) demonstrated that NPs-based miRNA delivery system, used to deliver miR-221, inhibits liver cancer cell proliferation and invasion in vitro as well as tumorigenesis in vivo.
On the whole, Varshney et al. (16) have set up an elegant and efficient system for the selective delivery of miR-199a-3p to liver tumor cells, able to significantly interfere with the tumoral process. The type of the nanoparticle system is relevant, as it is known that the NPs-cell surface interaction facilitates the internalization of NPs into targeting tumor cells (19). In addition, the choice to incorporate LA, the ligand for ASGPR, in the system has certainly increased HCC specificity as hepatoma cells overexpress ASGPR, in comparison with normal hepatocytes. Achieving the target is the most important issue in the use of therapeutic miRNA because the low stability of individual miRNA, subject to common nuclease degradation during passage in specific environments before its arrive on the site (20). High doses are usually required for miRNA-based therapies, which increase the risk for toxicities. A large number of studies reported that LA-modified delivery system could enhance the uptake of drugs and/or genes into hepatoma cells as a novel therapeutic strategy for HCC (21). RΔF-LA/miR NPs showed the highest concentration in HCC tissues with consequent antitumor activity an effect which was lost in absence of LA, its distribution concentrating predominantly in the lungs (RΔF/miR NPs) or in the kidney (naked miR).
The ability of LA to recognize its ASGPR target on tumoral hepatocytes made the system very efficient in increasing the molecule uptake in vitro and in vivo. Last but not least, RΔF-LA/miR NP displayed high biocompatibility, lack of toxicity, high stability and ease of synthesis. The injected mice did not show changes in bodyweight, tissue damage or, most importantly, significant immune response in comparison with mice treated with miR-199a-3p naked. Further studies should be devoted to test a range of doses of RΔF-LA/miR NPs for therapeutic potency, using a larger animal cohort than the present study.
Potential therapeutic implications of miR-199a-3p in HCC
The study by Varshney et al. (16) adds an important contribution to the field of therapeutic application of miRNA. So far, therapeutic application of miRNA (or its blockade) in humans has been limited to hepatitis C: the administration of miravirsen, a locked nucleic acid-modified phosphorothioate oligonucleotide targeting miR-122, resulted in a prolonged and dose-dependent decrease in hepatitis C virus (HCV) RNA, a very good proof of concept that therapeutic strategy can brilliantly work (22). This approach in antiviral therapy for HCV will probably not be developed further as the availability of direct acting antivirals, which rapidly and easily eliminate HCV infection in more than 95% of cases, has greatly overshadowed other therapeutic approaches. This is not such a case for HCC. Therapeutic options in HCC are still far from being satisfactory, especially in the high percentage of advanced patients in which curative treatment is not applicable already from presentation. In these cases, systemic targeted therapies have achieved so far only modest improvements in survival. Therefore, any new approach potentially able to block progression and increase patients’ survival would be welcomed.
A potential substantial drawback for this type of therapeutic approach in liver cancer is the molecular heterogeneity of HCC, due to the very diverse activation of pathways in the liver because of the inflammatory background of chronic liver disease. This means that it has not been so far identified a dominant molecular alteration driving liver carcinogenesis, but rather uncountable genetic or gene expression modifications, which prevent an easy blockade. From this point of view miRNA have an extremely favorable characteristic. An individual miRNA is able to control multiple targets and pathways therefore the manipulation of a single miRNA potentially inhibits the activity of a huge number of genes. Indeed, miR-199a-3p in HCC cells not only down-regulates mTOR expression, but also targets VEGFA, MMP2 and c-Met, which means influencing at the same time tumoral invasion, metastatic ability and angiogenesis of HCC cells. All these involved pathways make miR-199a-3p an attractive candidate as a therapeutic molecule.
There are still several issues that remain to be addressed. It will be necessary to investigate the possible long-term effects of treatment, in particular, the reversion of gene inhibition at the end of therapy. The goal will be to find a combination of molecules able to trigger a constitutive gene inhibition, in order to achieve a longstanding inhibition of tumoral growth.
Another attractive option, as suggested by the authors, is the possibility of combining delivery to the tumor site of miRNA coupled with anticancer drugs using the same NPs-based carrier system. This combination should be carefully explored, especially in HCCs characterized by a striking biological aggressiveness, as the aberrant activation of pathways suggests that a multi-level and multi-target block would be mostly appropriate (23). In these aggressive HCCs, there was a striking activation of neo-angiogenesis, driven by extremely elevated levels of angiopoietin-2, and at the same time, an up-regulated miRNA signature, involved in TGF-β/BMP-signaling pathway, angiogenesis, autophagy and inflammation (24). Most relevant, however, was the down-regulation of a single miRNA, mir-203a, associated with epithelial-mesenchymal transition, increased cell proliferation and angiogenesis. A combined approach, targeting mir-203 delivery to HCC cells, in order to reinstitute normal levels of expression and at the same time, a selective antiangiopoietin-2 blockade could provide a therapeutic option to patients with a very severe prognosis.
In conclusion, the study by Varshney et al. (16) suggests a very interesting and innovative approach for HCC therapy. So far, almost all studies using therapeutic delivery of miRNAs in HCC were performed in animal models (Table 1). Only one phase I study was performed in humans (25). In this study, MRX34, a double-stranded, synthetic version of miR-34a encapsulated in a liposomal nanoparticle (a liposomal miR-34a “mimic”), was administered to patients with solid tumors, of which 14 with HCC (Figure 1A). miR-34a was chosen as preclinical studies had indicated that it downregulates more than 30 oncogenes and it is critically under-expressed in many tumors. While the preliminary results seemed promising, the clinical application was terminated due to five immune-related serious adverse events. The system envisaged by Varshney et al. (16), being more selective for tumor cells and significantly less associated with stimulation of immune reactions, could be more advantageous for clinical use, at last allowing to transition from preclinical studies to clinical practice.
Table 1
| Reference | miRNA involved | Therapy approach: inhibition/replacement | Delivery system | Animal model or trial phase |
|---|---|---|---|---|
| Preclinical study | ||||
| Dhanasekaran et al.; Oncotarget 2017; doi: 10.18632/oncotarget.22342 | miRNA-17 | anti-miR-17 (RL01-17): inhibition | Lipid nanoparticle (LNP) | LAP-tTA/tet-O-MYC transgenic mice |
| Varshney et al.; Hepatology 2018; doi: 10.1002/hep.29643 | miRNA-199a/b-3p | miR-199a/b-3p mimics: replacement | Nanoparticle-based (NPs) | xenograft HCC mouse models |
| Li et al.; Intl J Nanomedicine 2018; doi: 10.2147/IJN.S157805 | miRNA-221 | miR-221 inhibitor: inhibition | Nanoparticle-based (NPs) | xenograft HCC mouse models |
| Fan et al.; Mol Ther Nucleic Acids 2017; doi: 10.1016/j.omtn.2017.03.010 | miRNA-375 | miR-375 and Doxorubicin co-delivery: replacement | Liposome nanoparticle | xenograft HCC mouse models |
| Fang et al.; J Hepatol 2015; doi: 10.1016/j.jhep.2015.05.008 | miRNA-188-5p | miR-188-5p mimics: replacement | Lentivirus-mediated | xenograft HCC mouse models |
| Zheng et al.; PLoS Genet 2015; doi: 10.1371/journal.pgen.1004873 | miRNA-101 | lent-miR-101: replacement | Lentivirus-mediated | orthotopic HCC mouse model |
| Hou et al.; Cancer Cell 2011; doi: 10.1016/j.ccr.2011.01.001 | miRNA-199a/b-3p | miR-199 mimics: replacement | Adeno-associated virus (AAV8) | xenograft HCC mouse models |
| Kota et al.; Cell 2009; doi: 10.1016/j.cell.2009.04.021 | miRNA-26a | miR-26 mimics: replacement | Adeno-associated virus | Myc-Induced HCC mouse |
| Clinical study | ||||
| Beg et al.; Invest New Drugs 2017; doi: 10.1007/s10637-016-0407-y | miRNA-34a | MRX34: replacement | Liposomal vehicle | Phase I |
HCC, hepatocellular carcinoma.
Acknowledgments
Funding: The authors’ work is supported by Regione Emilia-Romagna [Grants “PRU (Programma di ricerca Regione-Università) 2010-2012”] and by grant AI444-240 by Bristol-Myers-Squibb.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Meiyi Song (Division of Gastroenterology and Hepatology, Digestive Disease Institute, Tongji Hospital, Tongji University School of Medicine, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ncri.2018.08.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med 2009;60:167-79. [Crossref] [PubMed]
- Fornari F, Milazzo M, Chieco P, et al. MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res 2010;70:5184-93. [Crossref] [PubMed]
- Yin J, Hou P, Wu Z, et al. Circulating miR-375 and miR-199a-3p as potential biomarkers for the diagnosis of hepatocellular carcinoma. Tumour Biol 2015;36:4501-7. [Crossref] [PubMed]
- Liang Y, Ridzon D, Wong L, et al. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics 2007;8:166. [Crossref] [PubMed]
- Jia XQ, Cheng HQ, Qian X, et al. Lentivirus-mediated overexpression of microRNA-199a inhibits cell proliferation of human hepatocellular carcinoma. Cell Biochem Biophys 2012;62:237-44. [Crossref] [PubMed]
- Hou J, Lin L, Zhou W, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell 2011;19:232-43. [Crossref] [PubMed]
- Ren K, Li T, Zhang W, et al. miR-199a-3p inhibits cell proliferation and induces apoptosis by targeting YAP1, suppressing Jagged1-Notch signaling in human hepatocellular carcinoma. J Biomed Sci 2016;23:79. [Crossref] [PubMed]
- Ghosh A, Dasgupta D, Ghosh A, et al. MiRNA199a-3p suppresses tumor growth, migration, invasion and angiogenesis in hepatocellular carcinoma by targeting VEGFA, VEGFR1, VEGFR2, HGF and MMP2. Cell Death Dis 2017;8:e2706 [Crossref] [PubMed]
- Giovannini C, Fornari F, Dallo R, et al. MiR-199-3p replacement affects E-cadherin expression through Notch1 targeting in hepatocellular carcinoma. Acta Histochem 2018;120:95-102. [Crossref] [PubMed]
- Henry JC, Park JK, Jiang J, et al. miR-199a-3p targets CD44 and reduces proliferation of CD44 positive hepatocellular carcinoma cell lines. Biochem Biophys Res Commun 2010;403:120-5. [Crossref] [PubMed]
- Ke AW, Shi GM, Zhou J, et al. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma. Hepatology 2009;49:491-503. [Crossref] [PubMed]
- Kim JH, Badawi M, Park JK, et al. Anti-invasion and anti-migration effects of miR-199a-3p in hepatocellular carcinoma are due in part to targeting CD151. Int J Oncol 2016;49:2037-45. [Crossref] [PubMed]
- Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev 2015;81:128-41. [Crossref] [PubMed]
- Mohamed NK, Hamad MA, Hafez MZ, et al. Nanomedicine in management of hepatocellular carcinoma: challenges and opportunities. Int J Cancer 2017;140:1475-84. [Crossref] [PubMed]
- Panda JJ, Kaul A, Alam S, et al. Designed peptides as model self-assembling nanosystems: characterization and potential biomedical applications. Ther Deliv 2011;2:193-204. [Crossref] [PubMed]
- Varshney A, Panda JJ, Singh AK, et al. Targeted delivery of microRNA-199a-3p using self-assembled dipeptide nanoparticles efficiently reduces hepatocellular carcinoma in mice. Hepatology 2018;67:1392-407. [Crossref] [PubMed]
- D’Souza AA, Padma VD. Asialoglycoprotein receptor mediated hepatocyte targeting—Strategies and applications. J Control Release 2015;203:126-39. [Crossref] [PubMed]
- Li F, Wang F, Zhu C, et al. miR-221 suppression through nanoparticle-based miRNA delivery system for hepatocellular carcinoma therapy and its diagnosis as a potential biomarker. Int J Nanomedicine 2018;13:2295-307. [Crossref] [PubMed]
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 2008;7:771-82. [Crossref] [PubMed]
- Rüegger S, Großhans H. MicroRNA turnover: when, how, and why. Trends Biochem Sci 2012;37:436-46. [Crossref] [PubMed]
- Xue WJ, Feng Y, Wang F, et al. Asialoglycoprotein receptor-magnetic dual targeting nanoparticles for delivery of RASSF1A to hepatocellular carcinoma. Sci Rep 2016;6:22149. [Crossref] [PubMed]
- Janssen HL, Reesink HW, Lawitz EJ. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013;368:1685-94. [Crossref] [PubMed]
- Villa E, Critelli R, Lei B, et al. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a prospective study. Gut 2016;65:861-9. [Crossref] [PubMed]
- Critelli R, Milosa F, Faillaci F, et al. Microenvironment inflammatory infiltrate drives growth speed and outcome of hepatocellular carcinoma: a prospective clinical study. Cell Death Dis 2017;8:e3017 [Crossref] [PubMed]
- Beg MS, Brenner AJ, Sachdev J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs 2017;35:180-8. [Crossref] [PubMed]
Cite this article as: Milosa F, Faillaci F, Critelli R, Villa E. Lactosylated arginine-dehydrophenylalanine nanoparticles increase the selective delivery of miR-199a-3p to liver tumor cells enhancing antitumoral activity. Non-coding RNA Investig 2018;2:49.


