
MicroRNA-7: a potent suppressor of the malignant potential of hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the third-leading cause of cancer-related mortality worldwide (1). Although its early diagnosis can lead to curative treatment by operation or radiofrequency ablation (2), sorafenib, a multikinase inhibitor, has long been used for patients with advanced tumors and is the only drug available for treating advanced HCC (3). However, the clinical benefit of sorafenib is limited and almost all patients become rapidly refractory to therapy. Recently, two other multikinase inhibitors, regorafenib and lenvatinib, have become available (4), but sorafenib is still widely used worldwide. Thus, the development of novel therapeutic options against HCC, particularly against sorafenib-resistant HCC, is urgently needed.
Recently, tyrosine-protein kinase receptor 3 (TYRO3), a member of the TYRO3-AXL-MER family of transmembrane tyrosine kinase inhibitors, was found to be aberrantly expressed in HCC (5), particularly in HCC with more malignant behavior, as evidenced by higher levels of alpha-fetoprotein and a larger tumor diameter (5). Because TYRO3 modulates several oncogenic pathways (6) and induces neoplastic transformation in non-malignant cells (7), TYRO3 is being considered a therapeutic target for new HCC-targeted drug therapies.
Numerous reports have described the altered expression of miRNAs (miRs) in human HCC. For example, miRNA-7-5p (miR-7), which is downregulated in several human cancers, including HCC, is a representative tumor suppressor miRNA and regulates a number of oncogenic signal transduction pathways (8). Kabir et al. recently reported that sorafenib-resistant HCC exhibits aberrant TYRO3 expression, which can be overcome by miR-7 overexpression (9). In their study, the authors confirmed the potent growth-suppressive effects of miR-7 in the HCC cell line Huh7, both in vitro and in vivo. Subsequently, they identified TYRO3 as a novel target of miR-7. Knockdown of TYRO3 resulted in the reduction of Huh7 growth and the suppression of activation of the canonical PI3K/AKT pathway. Silencing of miR-7 using anti-miR-7 oligonucleotides induced the migration and invasion of Huh7 cells, effects that were antagonized by subsequent TYRO3 knockdown, which suggests that TYRO3 is a direct physiological target of miR-7 and plays a biologically crucial role.
The most interesting point in the Kabir et al. study is that knockdown of TYRO3 led to increased levels of the key pro-inflammatory cytokines CXCL10 and IL8, which contribute to growth, suggestive of a rebound surge. However, overexpression of miR-7 strongly suppressed CXCL10 and IL8 expression levels, and decreased TYRO3 levels, because those mRNAs contained seed sequences of miR-7, and thus could all be targeted. Therefore, miR-7 has broader antitumor effects than TYRO3. These results suggest that for clinical applications, precise pinpoint targeted therapeutics are not always superior to molecular agents with wider effects such as miR-7.
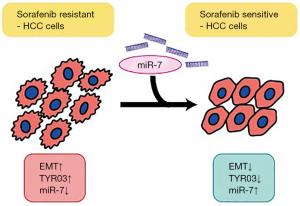
In addition, Kabir et al. found that in two sorafenib-resistant Huh7 sublines that had been established by culturing in increasing doses of sorafenib, the miR-7 expression levels were decreased and TYRO3 expression levels were increased. Intriguingly, although the two cell lines showed different growth rates (one more proliferative and the other less proliferative than the parental cells), both were more invasive and migrative, showing EMT characteristics. Knockdown of TYRO3 in these lines increased sorafenib sensitivity, but the effects were not synergistic. However, miR7 overexpression negated the EMT characteristics such as invasive and migrative behaviors, upregulated E-cadherin expression, reversed the effects of GAS6, a ligand for TYRO3 that induces migration and invasion, and showed synergistic effects with sorafenib. These novel findings provide interesting insights into the mechanisms of sorafenib-resistant HCC cells that involve the upregulation of TYRO3 and downregulation of miR-7 (Figure 1). The results shed new light on this field, simultaneously demonstrating the complexity of the phenomena.
Although this study is interesting in that it identifies the biological role of miR7 in the malignant behavior of HCC cells, issues that should be further considered are raised. One question is the regulatory mechanisms of miR-7 expression, as it is still unclear why miR-7 expression is downregulated in sorafenib-resistant HCC cells. Epigenetic changes may be induced in sorafenib-resistant clones or other intracellular signaling molecules may be altered. While such regulatory mechanisms may not be simple, their clarification could lead to more radical interventional methods to deploy against sorafenib resistance, in addition to the use of miR-7. Elucidation of the regulation of miRNA expression is a hot topic; thus, there is a need to further investigate the regulation of individual miRNAs as well as the general mechanisms.
Another question is the extent that miR-7 contributes to the sorafenib resistance of HCC cells. Because many miRNAs other than miR-7, such as miR-122 and let-7, are thought to be involved in sorafenib resistance (10), it is necessary to determine whether miR-7 is merely one of many, or is the most influential. In general, individual miRNAs are less potent than a combination; thus, the combined application of miRNA mimics or inhibitors may be necessary to fully revert sorafenib resistance. Thus, there is a need to summarize the available data and investigate combined supplementation for real-world clinical usage.
The third question is related to the regulation of miR function. Recent rapid progress in the area of non-coding RNAs has revealed new classes of regulators, such as circular RNAs, which regulate miRNA function. miR-7 is functionally regulated by the circular RNA, Cirs7 (11) In fact, in HCCs, Cirs7 expression levels are increased (12). Because the expression of Cirs7 impairs the function of miR-7, it may be interesting to examine determine if there are changes in miR-7 “function” (not “expression”) as well as Cirs7 levels in sorafenib-resistant cells, even if the miR-7 expression levels themselves are not changed.
The involvement of Gas6 in the Gas6-TYRO3 pathway is also intriguing. Because Gas6 is a potent ligand for TYRO3, neutralizing Gas6 may be beneficial against sorafenib-resistant HCC cells (13). Similarly, blocking antibodies against TYRO3 may also be useful against sorafenib-resistant HCC. Many hypotheses created due to the results of this study should be experimentally examined to determine their clinical significance.
Although several issues remain to be clarified to gain a full understanding of the reversion of sorafenib resistance in HCC cells, there is no doubt that the findings of Kabir et al. will shed new light on the development of novel therapeutics. The recent progress in RNA-based therapeutics (14,15), together with the report by Kabir et al., should lead to the development of novel therapeutic options that are based on completely different strategies from the canonical approaches. Further extensive studies of non-coding RNAs, including miRNAs, should help elucidate the pathogenesis of various diseases, leading to improvements in human health.
Acknowledgments
Funding: This work was supported by the Research Program on Hepatitis from Japan Agency for Medical Research and Development, AMED (to M Otsuka, #JP18fk0210214), and by the Project for Cancer Research And Therapeutic Evolution (P-CREATE) from AMED (to M Otsuka, #JP19cm0106602).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Meiyi Song (Division of Gastroenterology and Hepatology, Digestive Disease Institute, Tongji Hospital, Tongji University School of Medicine, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ncri.2018.05.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bertuccio P, Turati F, Carioli G, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol 2017;67:302-9. [Crossref]
- Costentin C, Layese R, Bourcier V, et al. Compliance With Hepatocellular Carcinoma Surveillance Guidelines Associated With Increased Lead-time Adjusted Survival of Patients With Compensated Viral Cirrhosis. Gastroenterology 2018; [Epub ahead of print]. [Crossref]
- Chuma M, Terashita K, Sakamoto N. New molecularly targeted therapies against advanced hepatocellular carcinoma: From molecular pathogenesis to clinical trials and future directions. Hepatol Res 2015;45:E1-11. [Crossref]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref]
- Duan Y, Wong W, Chua SC, et al. Overexpression of Tyro3 and its implications on hepatocellular carcinoma progression. Int J Oncol 2016;48:358-66. [Crossref]
- Graham DK, DeRyckere D, Davies KD, et al. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer 2014;14:769-85. [Crossref]
- Lan Z, Wu H, Li W, et al. Transforming activity of receptor tyrosine kinase tyro3 is mediated, at least in part, by the PI3 kinase-signaling pathway. Blood 2000;95:633-8.
- Fang Y, Xue JL, Shen Q, et al. MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology 2012;55:1852-62. [Crossref]
- Kabir TD, Ganda C, Brown RM, et al. A microRNA-7/growth arrest specific 6/TYRO3 axis regulates the growth and invasiveness of sorafenib-resistant cells in human hepatocellular carcinoma. Hepatology 2018;67:216-31. [Crossref]
- Kanthaje S, Makol A, Chakraborti A. Sorafenib response in hepatocellular carcinoma: MicroRNAs as tuning forks. Hepatol Res 2018;48:5-14. [Crossref]
- Peng L, Yuan XQ, Li GC. The emerging landscape of circular RNA ciRS-7 in cancer Oncol Rep 2015;33:2669-74. (Review). [Crossref]
- Yang X, Xiong Q, Wu Y, et al. Quantitative Proteomics Reveals the Regulatory Networks of Circular RNA CDR1as in Hepatocellular Carcinoma Cells. J Proteome Res 2017;16:3891-902. [Crossref]
- Yau TCC, Lencioni R, Sukeepaisarnjaroen W, et al. A Phase I/II Multicenter Study of Single-Agent Foretinib as First-Line Therapy in Patients with Advanced Hepatocellular Carcinoma. Clin Cancer Res 2017;23:2405-13. [Crossref]
- Fitzgerald K, White S, Borodovsky A, et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N Engl J Med 2017;376:41-51. [Crossref]
- Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N Engl J Med 2017;376:1430-40. [Crossref]
Cite this article as: Otsuka M, Tanaka E, Sekiba K, Seimiya T, Yamagami M, Koike K. MicroRNA-7: a potent suppressor of the malignant potential of hepatocellular carcinoma. Non-coding RNA Investig 2018;2:30.


